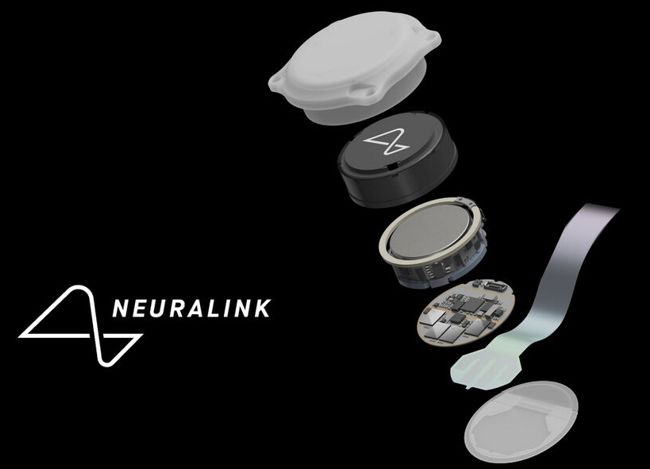
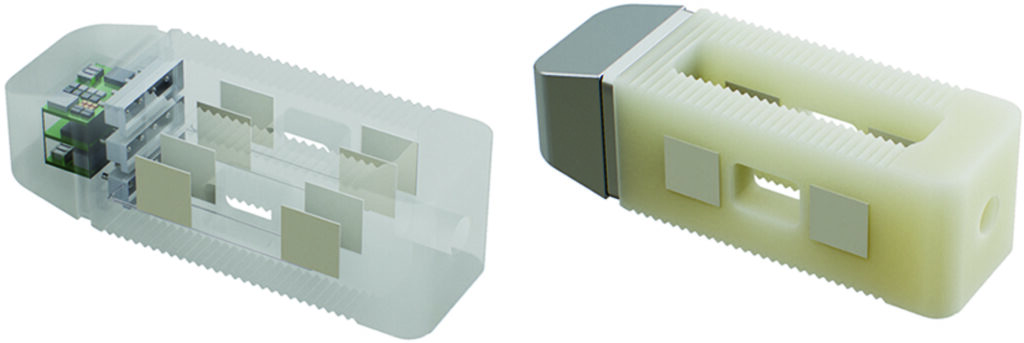
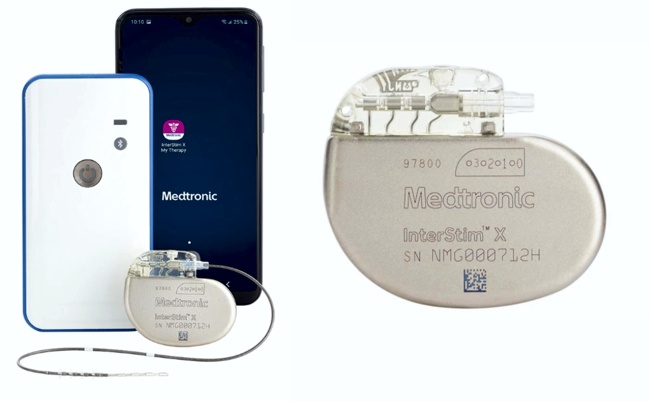
On July 18, 2023 Medtronic recalled approximately 384,000 ICD and CRT-D devices due to increased potential for a reduced- or no-energy HV therapy.
The devices, manufactured after July 17 have a glassed feedthrough, and its insulating layers may separate. When this happens, an unintended current pathway forms within the void created by the insulation separation, capable of conducting high levels of current during HV therapy. The low-energy output issue is most likely to appear when the device is programmed to deliver therapy in the AX>B pathway.