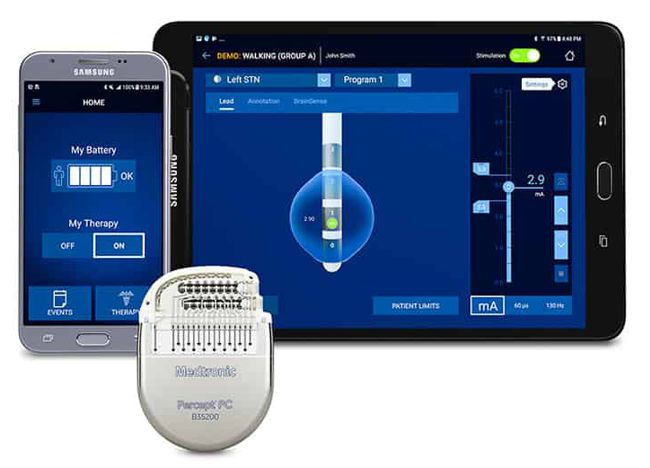
Image Credit: Medtronic
Medtronic announced that it received FDA approval for the Percept™ PC Deep Brain Stimulation (DBS) system.
According to the announcement:
“BrainSense™ technology makes Percept the first and only DBS neurostimulation system with the ability to chronically capture and record brain signals while delivering therapy to patients with neurologic disorders associated with Parkinson’s disease, essential tremor, dystonia, epilepsy or obsessive-compulsive disorder (OCD). Physicians can now track patient brain signals and correlate these with patient-recorded actions or experiences, such as symptoms, side-effects, or medication intake. This enables more personalized, data-driven neurostimulation treatment.
…
In addition to BrainSense technology, the Percept PC DBS system features several leading-edge innovations, including: