Impulse Dynamics (yes, the company for which I work) launched its new, CE-marked OPTIMIZER™ IVs implantable device for the treatment of heart failure at the 79th Annual Meeting of the German Cardiac Society (Mannheim, Germany, 3–6 April 2013).
The OPTIMIZER™ IVs Implantable Pulse Generator (IPG) is a programmable telemetric device intended to treat moderate to severe heart failure resulting from left ventricular dysfunction by monitoring intrinsic cardiac electrical activity and delivering Cardiac Contractility Modulation (CCM) signals during the ventricular absolute refractory period. These electrical signals are intended to influence myocardial properties in patients, thereby reducing their symptoms and improving quality of life and exercise tolerance.
The OPTIMIZER™ IVs IPG was designed to deliver CCM signals during the ventricular absolute refractory period in synchrony with locally-sensed electrical activity. The microprocessor controlled implantable device comprises intracardiac electrogram sensing circuits, control logic, communications circuitry, and circuitry to generate the CCM signals. Electrogram signals are detected from and CCM signals are delivered to the heart using standard chronically-implantable bipolar pacing leads. The implant procedure is similar to implantation procedures for other cardiac rhythm management devices.
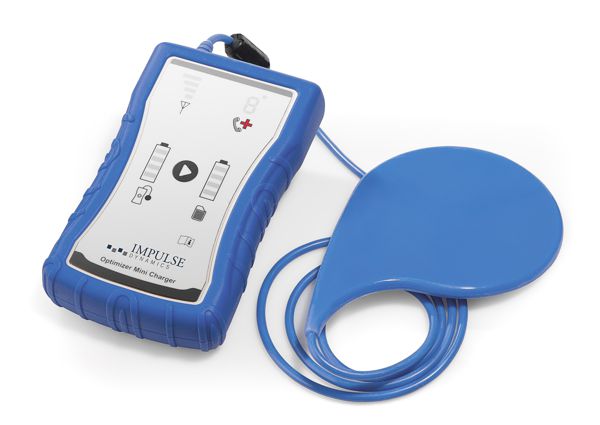
The OPTIMIZER™ IVs IPG is powered by an implantable-grade lithium-ion rechargeable cell. This chemistry is commonly used in implantable devices such as spinal cord stimulators and implantable ventricular assist devices. The battery is recharged via non-invasive, transcutaneous inductive energy transfer. The OPTIMIZER™ Mini Charger permits patients to recharge the battery of the IPG in the comfort of their home. When so required, the OPTIMIZER™ Mini Charger also warns the patient of changes in device state or CCM therapy delivery that require follow-up by a physician.
The OPTIMIZER™ IVs IPG is programmable, allowing attending medical personnel to program the value of the device’s parameters according to the patient’s needs. Programming of the implantable pulse generator is done through the OMNI II Programmer – a portable instrument with a friendly graphical user-interface, which provides attending medical personnel with all the information and controls required to control the IPG under a diversity of clinical settings.
The OPTIMIZER™ IVs System has been tested to demonstrate compliance with the applicable electrical safety and electromagnetic compatibility requirements. The OPTIMIZER IVs System is compliant with EN 45502, IEC 60601, and ISO 10993 as applicable to the device’s design and was designed in accordance with EN 62304 and ISO 14971. Impulse Dynamics has an ISO 13485-compliant quality system which is audited annually by a European Notified Body, and Impulse Dynamics is re-certified by the Notified Body every 3 years.
First human implants of the OPTIMIZER™ IVs were conducted in Germany on February 5, 2013 by Dr. Jürgen Kuschyk at the Universitätsklinik Mannheim and by Dr. Stephan Schmidt-Schweda at the Eichsfeld-Klinikum Worbis. The treatment cost for CCM therapy is covered both by statutory as well as private health insurances in Germany, Switzerland, Italy, and Austria.
Click here for the OPTIMIZER™ IVs product webpage (in German).
IMPORTANT NOTE: Please note that although I am employed by Impulse Dynamics, the purpose of this blog is in no way to promote or advertise the companies for which I work. As such, for any and all inquiries regarding Impulse Dynamics or its products, please contact Impulse Dynamics directly through its webpage at: www.impulse-dynamics.com
CAUTION – In the USA, the OPTIMIZER IVs System is an INVESTIGATIONAL DEVICE, LIMITED BY FEDERAL (OR UNITED STATES) LAW TO INVESTIGATIONAL USE. PLEASE READ FULL DISCLAIMER ON SIDE COLUMN AND IN THE LEGAL SECTION OF THIS WEBSITE.