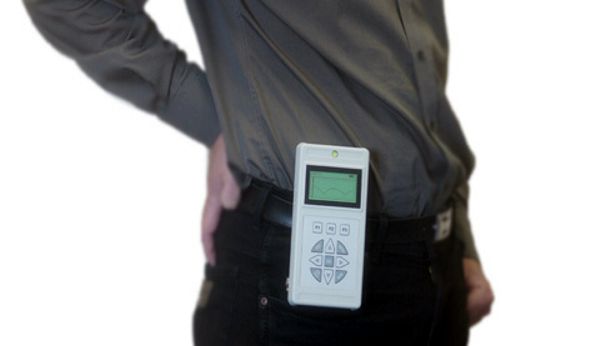
Pacesetter Human Tissue Stimulator (HTS) (c) David Prutchi, Ph.D., 2024
I recently acquired this device on eBay® (from a very kind seller, I may add). Although labeled as a “Rechargeable Pacemaker,” and looks just like the one at the Udvar-Hazy about which I had posted a few years ago, it is in fact a neurostimulator.
The Human Tissue Stimulator or “HTS” was developed subsequently to the rechargeable pacemaker, prompted by the clinical need for a rechargeable implantable neurostimulator.







 I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”:
I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”: