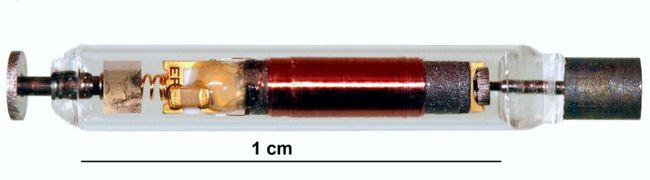
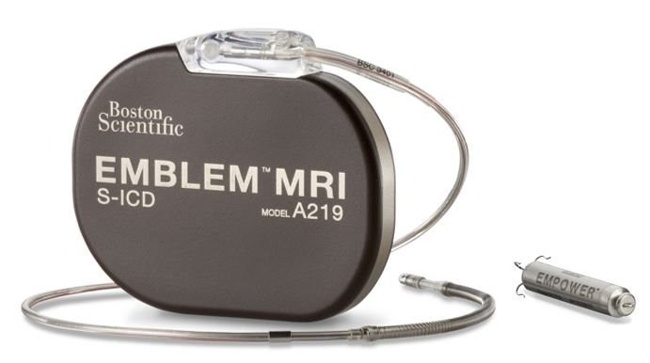
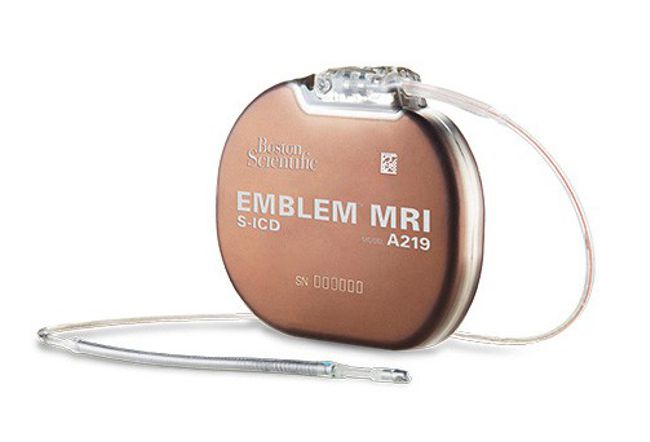
From: HFSA Scientific Statement: Update on Device Based Therapies in Heart Failure, ESTEP, JERRY D. et al., Journal of Cardiac Failure, 2024
The Heart Failure Society of America (HFSA) published today a new Scientific Statement titled “Update on Device Based Therapies in Heart Failure.” The statement was published online in the Journal of Cardiac Failure (JCF).
This statement discusses how novel device-based therapies, including CCM, BAT, and remote monitoring may bridge current gaps in HF treatment and outcomes. The statement also proposes a clinical pathway to implement FDA approved device-based therapies that align with current HF management workflow.