![IMAGE_Nanostim_Product_Shot[1]](https://www.implantable-device.com/wp-content/uploads/2013/10/IMAGE_Nanostim_Product_Shot1.jpg)
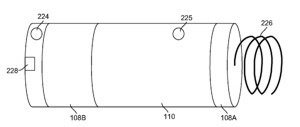
St. Jude today announced the first U.S. implant in the company’s LEADLESS II pivotal trial designed to evaluate the Nanostim™ leadless pacemaker for FDA approval. The world’s first retrievable, non-surgical pacemaker was implanted at The Mount Sinai Hospital in New York City by Dr. Vivek Reddy. The LEADLESS II pivotal trial is a prospective, non-randomized, multi-center, international clinical research trial designed to evaluate the safety and effectiveness of the Nanostim leadless pacemaker in patients indicated for the device. It is being conducted under an Investigational Device Exemption (IDE) from the FDA, and will enroll approximately 670 patients at 50 centers in the U.S., Canada and Europe.
According to the press release:
The Nanostim leadless pacemaker is designed to be placed directly in the heart without the visible surgical pocket, scar and insulated wires (called leads) required for conventional pacemakers. Implanted via the femoral vein with a steerable catheter, the device offers physicians the same pacing therapy through a less-invasive approach as compared to traditional pacemaker procedures that require more extensive surgery. The device is designed to be fully retrievable, so that it can be readily repositioned throughout the implant procedure and later retrieved if necessary.