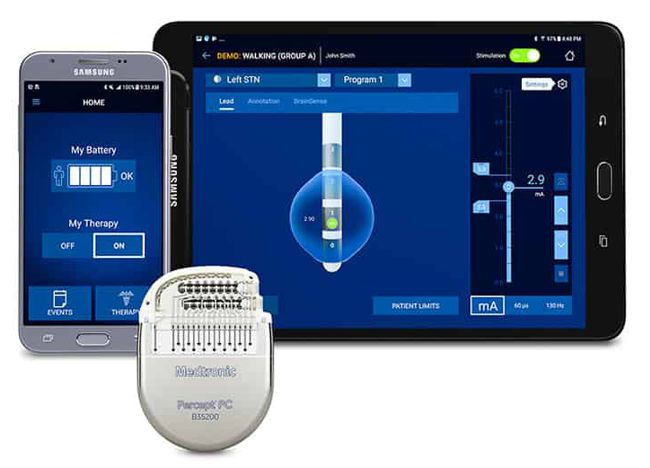
Image Credit: Medtronic
Medtronic announced that it received FDA approval for the Percept™ PC Deep Brain Stimulation (DBS) system.
According to the announcement:
“BrainSense™ technology makes Percept the first and only DBS neurostimulation system with the ability to chronically capture and record brain signals while delivering therapy to patients with neurologic disorders associated with Parkinson’s disease, essential tremor, dystonia, epilepsy or obsessive-compulsive disorder (OCD). Physicians can now track patient brain signals and correlate these with patient-recorded actions or experiences, such as symptoms, side-effects, or medication intake. This enables more personalized, data-driven neurostimulation treatment.
…
In addition to BrainSense technology, the Percept PC DBS system features several leading-edge innovations, including:
-
The only DBS system eligible for 3T and 1.5T full-body MRI scans, providing patients access to cutting-edge medical imaging
-
Smart battery for personalized prediction of remaining battery life providing elevated peace of mind while planning for device replacement
-
Improved battery longevity compared to Medtronic’s Activa™ PC neurostimulator (when using similar settings and functionality) in a smaller (reduced volume), ergonomic design for patient comfort
-
Low pulse width (duration of the pulse), providing expanded stimulation options
-
Enhanced Patient Programmer leveraging a user-friendly, custom-configured Samsung mobile device that allows patients to manage their therapy easily
-
Designed to facilitate expanded capabilities in the future via software upgrades – to prepare for what’s next in DBS”
