
The 2015 “Made in China 2025” and subsequent domestic development initiatives have significantly benefited Chinese medical device manufacturers. The 2015 plan prioritized the expansion of high-tech device production with the goal of supplying 70% of China’s device market by 2025. More recently, in 2021, the Chinese government issued the 14th five-year plan (2021–25) that aims at making at least six Chinese companies reach the top 50 revenue-making medical device companies globally.
I was recently chatting with Victor Pikov at the First AIMD Workshop, and he mentioned that in the last couple of years the number of Chinese AIMD manufacturers have grown from 5 to over 10. I was really surprised about the progress that China has made in the AIMD field, so this blog expands on the list of Chinese AIMD companies that Victor compiled and kindly shared with me.
Shaanxi Qinming Medical Instrument Co. Ltd. (Subsidiary of Lepu)

Image Credit: Qingmin
Headquarters: Baoji
Founded: 1985
Implantable Products: Pacemakers, pacing leads
Website: https://en.lepumedical.com/products/pacemakers-and-leads/
During 1985, US-based Cardio-Pace Medical based entered into an agreement with Qinling Semiconductor to establish Qinming Medical, Inc., a Sino-American joint venture in Baoji, China, for the manufacture and distribution of cardiac pacemakers and accessory products. In 1992, Cardio-Pace sold its 49% interest in the joint venture to Qinming.
In 2010, Lepu Medical of Beijing purchased a 30.46 percent stake in Qinming as part of its efforts to forge an integrated Chinese cardiovascular products company. In 2013, Lepu completed the acquisition of Shaanxi Qinming Medical Instruments Co., Ltd.
Around that time, Qinming developed the QM7211, which was the first domestically developed dual-chamber rate-responsive pacemaker in China. Its clinical study to evaluate it for non-inferiority against a Biotronik Talos pacemaker was successfully completed in 2015.
Today, Qinming is Lepu’s subsidiary in charge of the development, manufacture, and marketing of pacemaker products. Their line includes single- and dual-chamber devices, as well as passive and active-fixation leads.
LEPU Medical Technology

Image Credit: Lepu Medical Technology (Beijing) Co., Ltd.
Headquarters: Beijing
Founded: 1999
Implantable Products: CCM, pacemakers (?)
Website: https://en.lepumedical.com/
Although Qinming is Lepu’s subsidiary in charge of the development, manufacture, and marketing of pacemaker products, it is rumored that Lepu is developing its own line of pacemaker products. Lepu recently announced that it had started clinical implants of its CCM device.
LifeTech Scientific

Image Credit: Lifetech Scientific
Headquarters: Shenzhen
Founded: 1999
Implantable Products: Pacemakers, pacing leads (based on Medtronic technology)
Website: https://www.lifetechmed.com/en/
In October 2012 Medtronic invested in LifeTech, with the agreement calling for LifeTech to develop a line of pacemakers and leads using its manufacturing plant in Shenzhen, with Medtronic supplying technology, training and support. In 2021, Medtronic and LifeTech extended the agreement on the HeartTone pacemaker project to develop Chinese-made MRI-conditional pacemakers.
Nurotron Biotechnology Co. Ltd. 浙江诺尔康神经电子科技股份有限公司
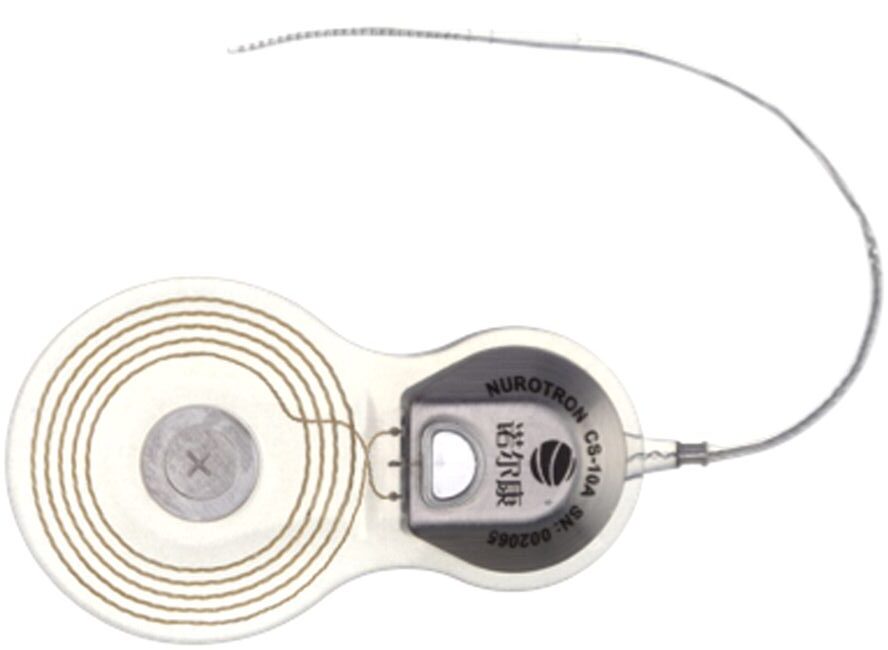
Image Credit: Nurotron
Headquarters: Hangzhou
Founded: 2006
Implantable Products: Cochlear implant systems
Website: https://www.nurotron.com/
Zhejiang Nurotron Biotechnology Co., Ltd was funded in 2006 to develop cochlear implant systems. Their current products on the market are “Venus” and “Enduro” cochlear implant systems.The company’s headquarters and production base are in Hangzhou, China, and with a research and development center in California.
PINS Medical 品驰医疗

Image credit: PINS
Headquarters: Beijing
Founded: 2008
Products: Neurostimulators for SCS, DBS, VNS, Sacral Nerve Stimulation; SCS, DBS, and VNS leads
Website: http://m.pinsmedical.com/en/
PINS Medical was founded in 2008 as part of the “National Engineering Laboratory for Neuromodulation”. It cooperates closely with Tsinghua University.
SceneRay 苏州景昱医疗器械有限公司

Image Credit: Sceneray
Headquarters: Suzhou
Founded: 2009
Implantable Products: Neurostimulators for DBS, DBS leads
Website: https://www.sceneray.com/
SceneRay was established in 2009 at the Biobay Industrial Park in Suzhou. It develops and manufactures neuromodulation devices for deep-brain stimulation.
Rishena Medical 常州瑞神安医疗器械有限公司

Image Credit: Rishena Medical
Headquarters: Changzhou
Founded: 2013
Implantable Products: Neurostimulators for VNS and SCS, VNS and SCS leads
Website: https://www.rishena.com
Rishena Medical was started as a project to develop implantable stimulators based on a platform designed by the Institute of Microelectronics of Tsinghua University /Shenzhen Tsinghua University Research Institute. The company was registered in 2013 and set up a factory in the Changzhou National Development Zone, which included a 100,000-level cleanroom. NMPA approval for its products include 2020 for the VNS neurostimulator, and 2022 for SCS. Rishena is currently conducting a clinical trial on its closed-loop brain stimulator (CNS) for Parkinson’s and epilepsy.
MicroPort Sorin CRM (Shanghai) Co

Image Credit: MicroPort
Headquarters: Shanghai
Founded: 2015
Implantable Products: Pacemakers (made in China, with other CRM products made in France, and pacing leads made in the Dominican Republic)
In 2015 MicroPort Sorin CRM (Shanghai) Co was established as a joint venture with LivaNova. In 2018, MicroPort and LivaNova closed the sale of LivaNova’s cardiac rhythm management business for $190M. MicroPort thus established MicroPort with global headquarters in Clamart, near Paris, France and an associated company of MicroPort.
Singular Medical
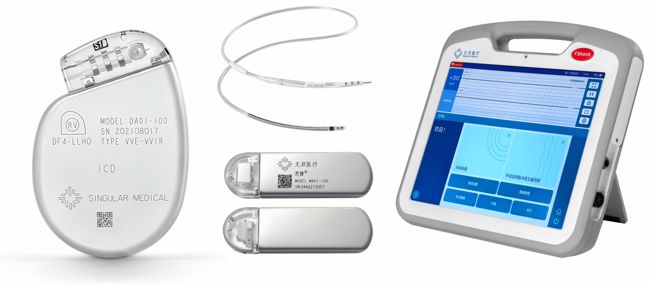
Image Credit: Singular Medical
Headquarters: Suzhou
Founded: 2017
Implantable Products: ICD, ILR, active-fixation DF-4 lead
Website: https://www.singularmedical.net
Singular Medical was founded in 2017 and is headquartered in Suzhou, China. They have development offices in Beijing, Irvine CA, and Minneapolis MN.
In May 2023 they completed the first Chinese human clinical trial on a domestic ICD, which puts them on their way to marketing their single-chamber, DF-4 ICD. According to their website, the device offers tiered therapy and Bluetooth-based telemetry, and the programmer is an Android-based tablet PC. The website also shows an active-fixation DF-4 single-coil lead, as well as an ILR.
Genlight 杭州佳量医疗科技有限公司

Image Credit: Genlight
Founded: 2020
Headquarters: Hangzhou
Implantable Products: DBS neurostimulator for epilepsy
Genlight MedTech was founded in 2020 in Hangzhou to develop technology platforms in brain-computer interface, medical lasers, and brain-computer probes. Developed products include a magnetic resonance-guided laser ablation system, an implantable closed-loop responsive neurostimulation system for epilepsy and Parkinson’s disease, as well as a SEEG electrode.
Sneuro Medical (Seeneuro) 杭州神络医疗

Image Credit: Seeneuro
Headquarters: Hangzhou
Founded: 2018
Implantable Products: Neurostimulator for SCS
Hangzhou Shenluo Medical Technology Co., Ltd. (SNeuro or Seeneuro) was founded in 2018 to develop and produce implantable neurostimulation and brain-computer interface devices. It has built nearly 1,000 square meters of 100,000-level GMP factories and 10,000-level laboratories. Intends to expand its offerings to include fully implantable peripheral nerve stimulators and autonomic nerve stimulators.
General Stim – 杭州承诺医疗科技有限公司

Image Credit: General Stim
Headquarters: Hangzhou
Founded: 2013
Implantable Products: Sacral nerve stimulator
General Stim received NMPA’s approval for their implantable sacral nerve stimulator in June of 2023. General Stim claims that it is meant for the treatment of overactive bladder, urinary retention, fecal incontinence, constipation and other lower urinary tract and pelvic floor disorders.
Leadinno Medical Valley Co. Ltd. 北京领创医谷科技发展有限责任公司
Headquarters: Beijing
Founded: 2016
Implantable Products: SCS and PNS
Website: https://www.leadinno.cn/
In September 2022, Beijing-based LeadInno Medical Valley announced that it had received $13.6M of Series A+ financing l for the R&D and production of spinal cord stimulation (SCS) and peripheral nerve stimulation (PNS) products.
CoreRhythm Medical Technology 杭州丹源医学科技 (Danyuan Medical)
Headquarters: Hangzhou
Founded: 2018
Products: Pacemakers
Website: http://www.danyuan-med.com/
It is unknown if CoreRhythm (Danyuan Medical) is currently active.
Super Vision
Headquarters: Beijing
Founded: 2018
Products: Muscular functional electrical stimulator for the treatment of nystagmus

Image Credit: Super Vision
Super Vision was founded in Beijing in 2018 by professors from the Peking University and Beijing University of Technology. It developed the i-NYS implantable stimulation device for the treatment of congenital nystagmus.
LVADs
Besides AIMDs, the number of Chinese LVAD and artificial heart companies has also been growing. These are the ones I’ve found:
CH Biomedical (Tongxin Medical Equipment)

Image Credit: CH Biomedical (BrioHealth Solutions)
Headquarters: Suzhou
Founded: 2008
Products: LVAD (magnetically-levitated)
Website: https://www.chbiomedical.com
https://briohealthsolutions.com
In November 2021, CH-VAD, made by Suzhou Tongxin Medical Equipment, with Chinese-developed magnetic levitation technology was approved by NMPA.
Yongrenxin

Image Credit: Yongrenxin
Headquarters: Chongqing
Founded: 2014
Products: LVAD (hydrodynamic levitation)
The Yongrenxin LVAD project was established as a joint venture with Japan in 2014. The device’s clinical trial started in 2018 with 15 successful implants. In September 2019 the EVAHEART I (Yongrenxin artificial heart) developed and produced by Chongqing Yongrenxin Medical Instrument Co., Ltd. was approved by the NMPA.
Aerospace Taixin Technology Co., Ltd.
Headquarters: Tianjin
Products: LVAD (?)
Core Medical Technology Co., Ltd.

Image Credit: Core Medical
Headquarters: Shenzhen
Founded: 2016
Products: Corheart 6 miniaturized magnetically-levitated LVAD
Website: https://www.coretechmed.com
Rocor Medical Technology
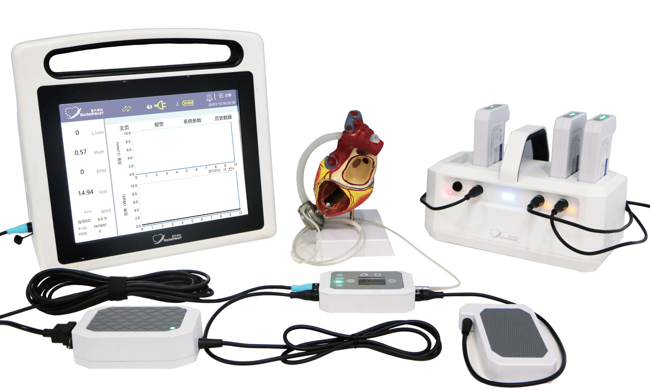
Image Credit: Rocor Medical
Headquarters: Tianjin
Products: LVAD (magnetohydrodynamic levitation)
The HeartCon or “rocket heart” LVAD was independently developed and is produced by Rocor Medical Technology Co. It was approved by NMPA in July 2022.
