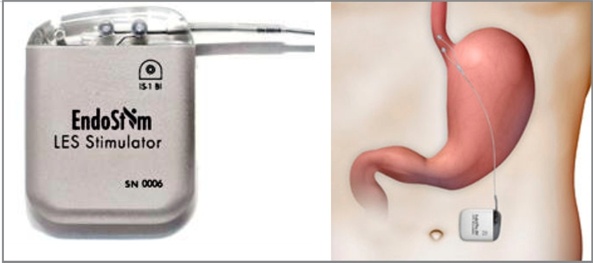
EndoStim was a medical device company based in Dallas, Texas, and Nijmegen, The Netherlands, developing and commercializing a treatment for gastroesophageal reflux disease (GERD).
On October 15, 2019 EndoStim terminated its Clinical Investigation of the EndoStim® Lower Esophageal Sphincter (LES) Stimulation System due to lack of funding. According to a letter sent by EndoStim to its investigators, clinical and technical support will no longer be available for the devices after January 14, 2020
EndoStim terminated operations and entered into an Assignment for Benefit of Creditors under Delaware law, which is a state law liquidation procedure similar to a Chapter 7 bankruptcy.
EndoStim’s neurostimulation technology was designed to provide long-term reflux control by restoring normal esophageal function. The therapy directly targeted the patient’s weak or dysfunctional lower esophageal sphincter (LES) muscle between the stomach and the esophagus, often the underlying cause of reflux. The system consisted of an implantable neurostimulator and a lead that delivered low energy stimulation to the LES. The EndoStim procedure was designed to preserve natural anatomy in order to reduce or avoid typical gastrointestinal side effects of traditional anti-reflux surgery.
EndoStim’s Website is at: http://www.endostim.com
