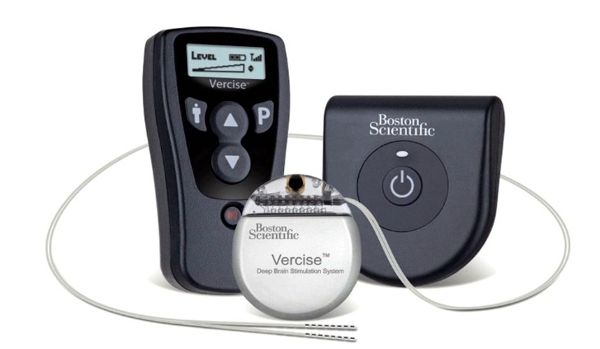
St. Jude Medical announced CE Mark approval of its Brio™, Libra™ and LibraXP™ deep brain stimulation (DBS) systems for managing the symptoms of intractable primary and secondary dystonia, a neurological movement disorder that causes a person’s muscles to contract and involuntarily spasm, reducing the ability to control movement. This approval represents the first by a regulatory agency for the use of deep brain stimulation to manage both primary and secondary dystonia.