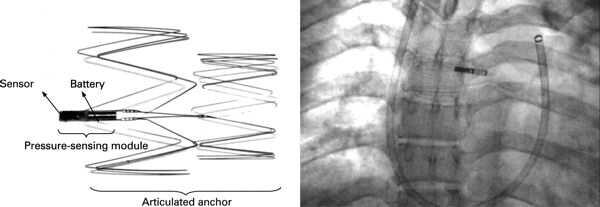
Intec AID-B. (c) 2021 David Prutchi, PhD.
Conceived by physicians Michel Mirowski and Morton Mower, and supported by Dr. Stephen Heilman, the first automatic implantable defibrillator was developed and manufactured under the name of “AID” by Medrad/Intec Systems in Pittsburgh, PA.
It was encased in titanium and hermetically sealed with a laser beam weld. It had a volume of 145 ml and weighed 250 gm. The device was powered by lithium batteries which had a projected monitoring life of 3 years or a discharge capability of 100 shocks.
The ICD was designed to detect ventricular fibrillation and sinusoidal ventricular tachycardia, and it attempted to using a truncated exponential 25 Joules pulse. The device could recycle three times during a single episode with the third and fourth pulses delivered at 30 Joules.
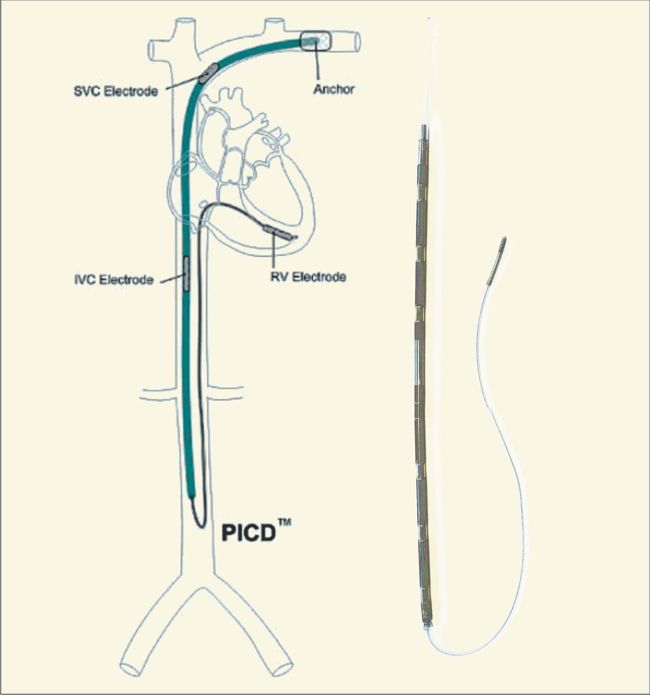
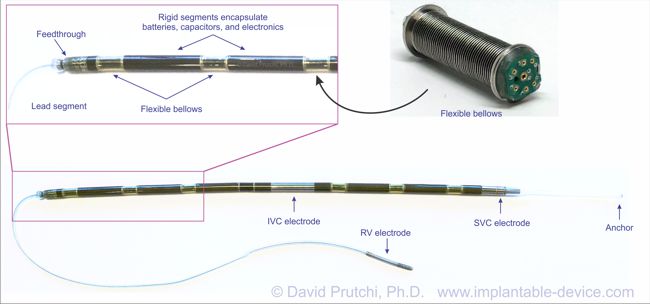
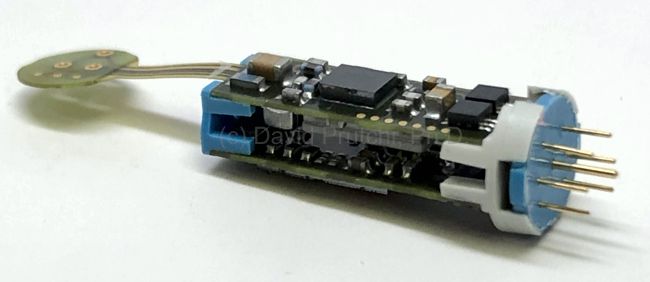
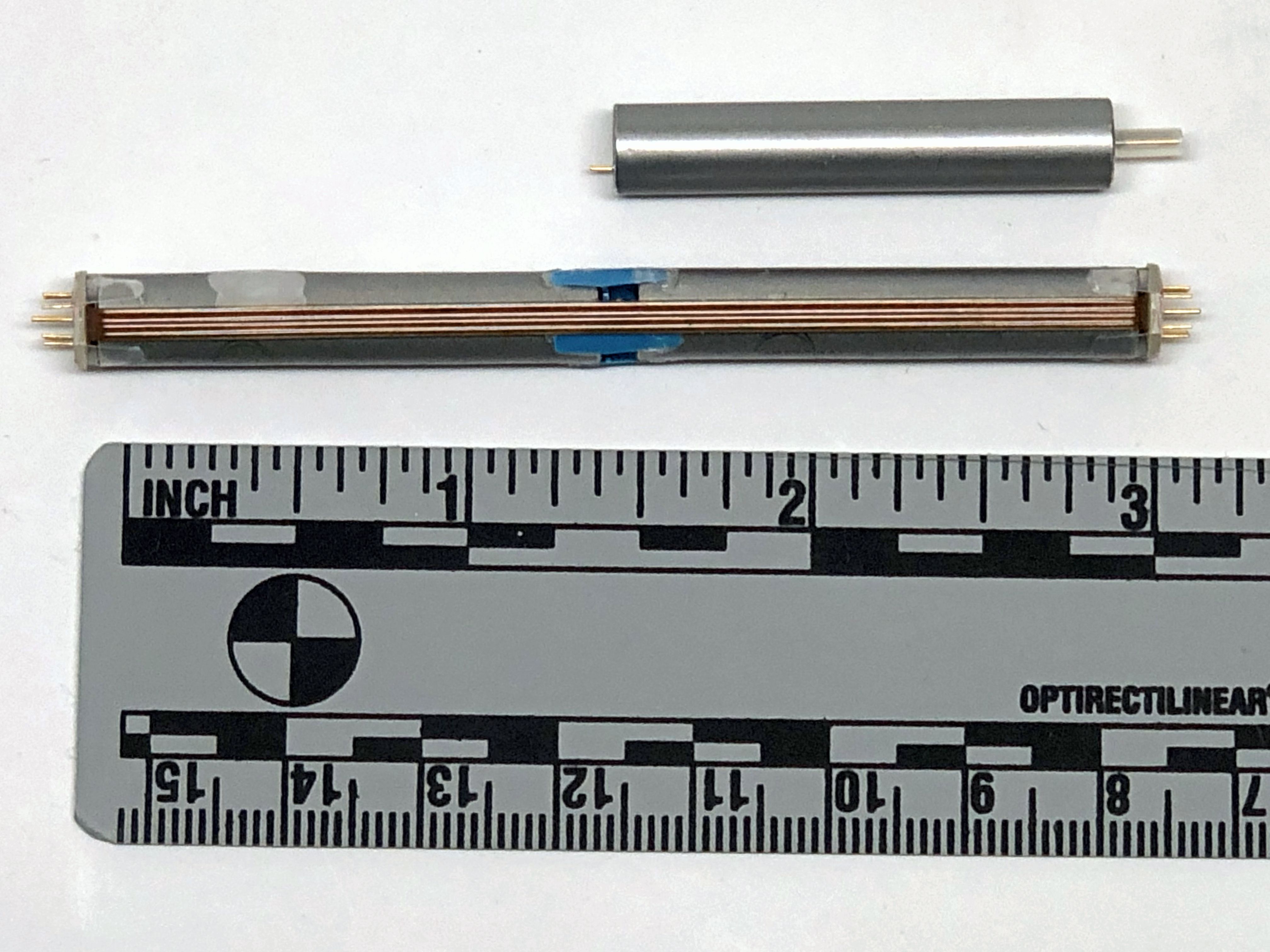
The device was meant to be connected to electrodes made from titanium and coated with silicone rubber. One defibrillating electrode was located intravascularly in the superior vena cava (SVC) at the right atrial junction. The second electrode had the configuration of a rectangular patch or a cup and was positioned over the cardiac apex.
First implants of the device were conducted at the Johns Hopkins Hospital in Baltimore in 1980. These devices were not programmable and manufactured on an individual basis. The AID was then improved with rate-counting circuitry and could synchronize shocks with the R-wave. Efforts were also made to reduce the recycling time after a failed shock. The new device was called the AID-B (shown in the picture), but was still manufactured according to individual specifications.
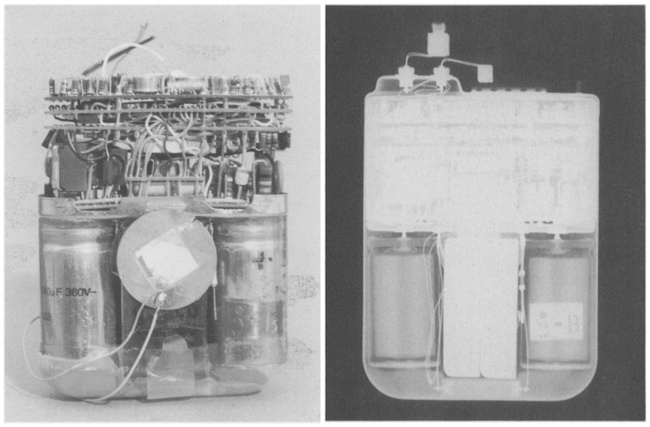
Circuitry of the AID-B implantable defibrillator. From: PJ Troup, “Implantable Cardioverters and Defibrillators”, Current Problems in Cardiology, Volume 14, Issue 12, December 1989.







 I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”:
I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”:


 In 1965, Australian medical device pioneer Noel Gray established Telectronics – Australia’s first manufacturing facility for producing pacemakers that were designed in-house. Telectronics was an innovative developer, achieving some major successes in the early cardiac pacing field, for example, Telectronics’ leads allowed narrowing the pacing pulse to its current nominal of 0.5 milliseconds; encapsulating the pacemaker in titanium instead of epoxy; using a microplasma weld to join the two halves of the pacemaker capsule; creating one of the first rate-responsive ‘demand’ pacemakers; and isolating the pacemaker’s battery in a separate compartment to deal with the problem of leaking mercury-zinc batteries.
In 1965, Australian medical device pioneer Noel Gray established Telectronics – Australia’s first manufacturing facility for producing pacemakers that were designed in-house. Telectronics was an innovative developer, achieving some major successes in the early cardiac pacing field, for example, Telectronics’ leads allowed narrowing the pacing pulse to its current nominal of 0.5 milliseconds; encapsulating the pacemaker in titanium instead of epoxy; using a microplasma weld to join the two halves of the pacemaker capsule; creating one of the first rate-responsive ‘demand’ pacemakers; and isolating the pacemaker’s battery in a separate compartment to deal with the problem of leaking mercury-zinc batteries.