
Image Credit: NeuroPace
NeuroPace is a privately-held company in Mountain View, CA. Their RNS® implantable stimulator, along with depth leads and cortical strip leads are designed for the treatment of medically refractory partial epilepsy.
Unlike Cyberonics’ VNS IPGs, the RNS neurostimulator is designed to detect abnormal electrical activity in the brain and respond by delivering electrical stimulation to normalize brain activity before the patient experiences seizure symptoms. The neurostimulator is implanted in the cranium and connected to one or two leads that are implanted near the patient’s seizure focus.
NeuroPace recently disclosed that it has raised about $18 million of a planned $50 million in new funding. NeuroPace has raised about $180 million since it was founded in 1997.


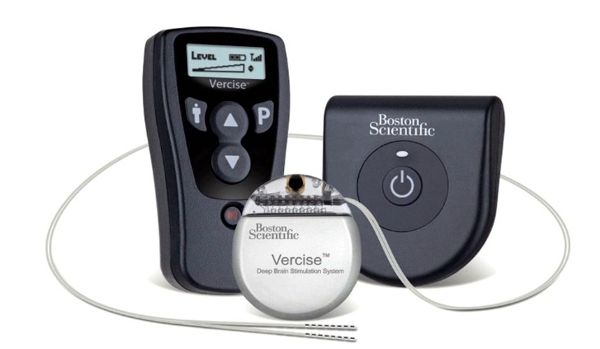

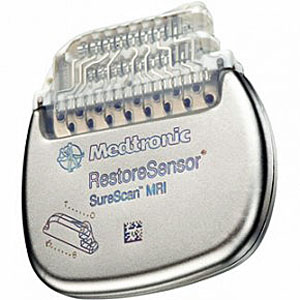
 Toronto-based
Toronto-based 
 Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.
Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.


