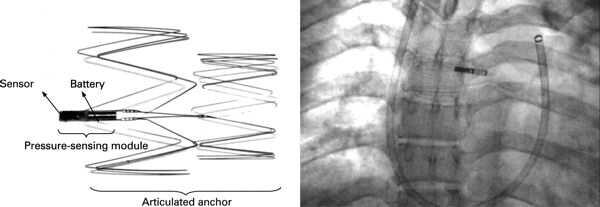
Remon Medical Technologies, Ltd. was founded in 1997 in Caesarea, Israel to develop implantable, wireless pressure sensors.
Remon developed an implantable hemodynamic monitor, which allowed on-demand, non-invasive, leadless self-monitoring of pulmonary artery pressure by the patient at home. ImPressure devices were placed in the pulmonary artery, and transmitted pressure readings to a hand-held monitor. It was hoped that the system would provide early warning of the need for treatment, avoiding hospitalization and deterioration in the patient’s condition.
 The Remon ImPressure/RemonCHF sensor was implanted via right-heart catheterization using jugular or femoral access. The implant was advanced to the desired site of implantation using a delivery sheath and was released like a self-expandable stent.
The Remon ImPressure/RemonCHF sensor was implanted via right-heart catheterization using jugular or femoral access. The implant was advanced to the desired site of implantation using a delivery sheath and was released like a self-expandable stent.
The device used acoustic waves for activation and data transfer, and produces a curve corresponding to dynamic pressure variations. The primary drawback of this device was its use of a piezoelectric-based pressure-sensing element, which can only measure dynamic and not static pressures.
From a 2004 press release:
“The Company’s core technology utilizes acoustic waves, which both energize and communicate with the implanted device. The advantage of this approach is that acoustic waves transmit effectively inside the body (through soft tissue, bones and fluids), and are not absorbed by the intervening tissue. Acoustic communication requires very little energy to achieve a high signal-to-noise ratio when accessing locations deep inside the body.
The implant is energized and activated on-demand via an external transducer. The implant converts the acoustic waves into electrical energy via its proprietary energy exchanger.
As the system’s internal transducer operates at a low resonance frequency, Remon’s implant is omni-directional, insensitive to the exact direction of the external transducer.”
In August 2007, Remon was acquired by Boston Scientific Corporation. As of April 2011, patients that had previously been implanted with the device were being followed up under the “Monitoring Pulmonary Artery Pressure by Implantable Device Responding to Ultrasonic Signal (PAPIRUS III)” study.
