Image Credit: Medtronic
Medtronic announced that has received U.S. Food and Drug Administration (FDA) approval for the CareLink SmartSync(TM) Device Manager. With the introduction of SmartSync, physicians will now be able to use an Apple® iPad® to program and manage data from Medtronic’s BlueSync-enabled implanted cardiac devices.
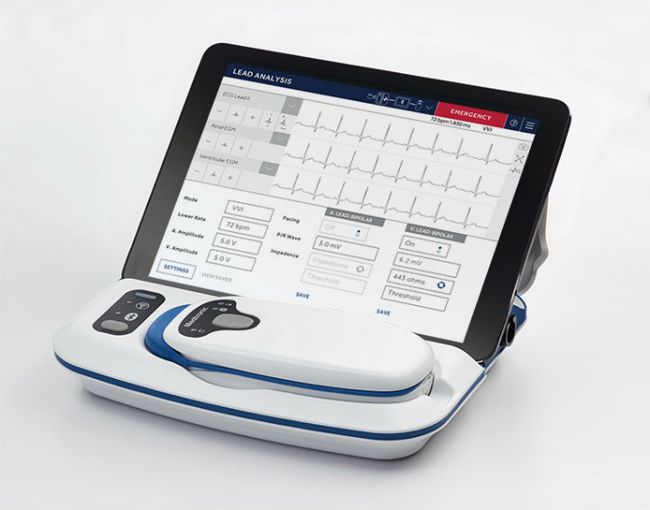
SmartSync is Medtronic’s next-generation programmer and pacing system analyzer. It features a simplified user interface, enhanced security and Bluetooth® capabilities to communicate with compatible cardiac devices.
The new CareLink SmartSync Device Manager (programmer) includes:
- Device manager app running on an iPad or Android tablet. This is the primary interface of the device manager. From the device manager app, one can access the implantable device app and CareLink SmartSync analyzer.
- Patient Connector (telemetry head), which is used to interrogate and program implantable devices. It is used with the Medtronic apps to interrogate, analyze, and/or program implantable Medtronic devices. The patient connector uses Bluetooth technology to transmit data to a Medtronic app for further processing
- The Medtronic Model 24970A base, which provides analyzer and ECG hardware functionality during device implant, invasive troubleshooting, and implanted device follow-up procedures. The base also provides a charge cradle and USB port to charge the patient connector, and the USB port is used to transmit data between the patient connector and the device manager app.

Image Credit: Medtronic
