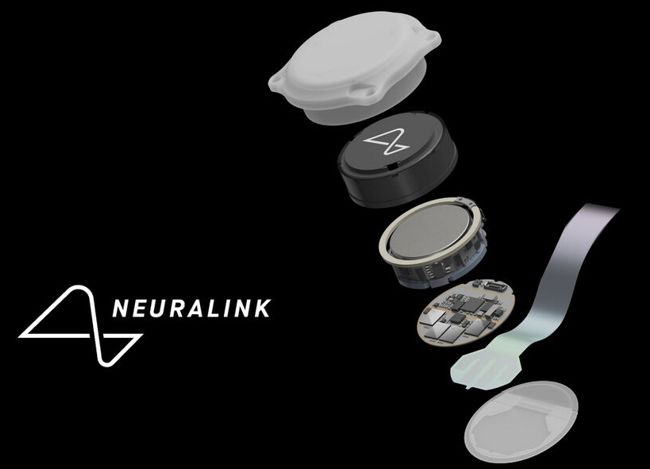
Image credit: Neuralink
Neuralink announced that they have started recruiting subjects with quadriplegia due to cervical spinal cord injury or amyotrophic lateral sclerosis (ALS) for their PRIME Study (short for Precise Robotically Implanted Brain-Computer Interface), which is an IDE trial for their fully-implantable, wireless brain-computer interface (N1) and surgical robot (R1). The study will assess the initial functionality of Neuralink’s BCI for enabling people with paralysis to control external devices with their thoughts.
According to the announcement:
During the study, the R1 Robot will be used to surgically place the N1 Implant’s ultra-fine and flexible threads in a region of the brain that controls movement intention. Once in place, the N1 Implant is cosmetically invisible and is intended to record and transmit brain signals wirelessly to an app that decodes movement intention. The initial goal of our BCI is to grant people the ability to control a computer cursor or keyboard using their thoughts alone.
The PRIME Study is being conducted under the investigational device exemption (IDE) awarded by the FDA in May 2023 and represents an important step in our mission to create a generalized brain interface to restore autonomy to those with unmet medical needs.
Those who have may qualify.
