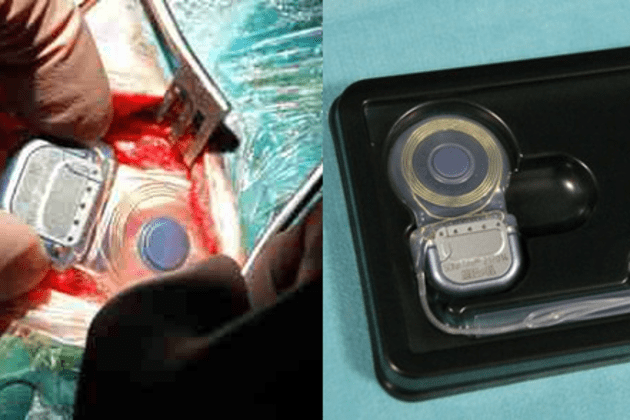
On Sept. 28, 2012 Boston Scientific announced that the FDA granted regulatory approval for its S-ICD® System, the world’s first and only commercially available subcutaneous implantable defibrillator (S-ICD) for the treatment of patients at risk for sudden cardiac arrest (SCA). The S-ICD System sits entirely just below the skin without the need for leads to be placed into the heart.









 Yesterday Boston Scientific announced financial results for the first quarter ended March 31, 2012. Sales of Cardiac Rhythm Management devices were $501M vs. $559M for Q1 a year ago, or a decrease of 10%. Sales of Neuromodulation devices increased by 8% a year ago from $77M to $84M for Q1.
Yesterday Boston Scientific announced financial results for the first quarter ended March 31, 2012. Sales of Cardiac Rhythm Management devices were $501M vs. $559M for Q1 a year ago, or a decrease of 10%. Sales of Neuromodulation devices increased by 8% a year ago from $77M to $84M for Q1. St. Jude Medical today
St. Jude Medical today 

 Medtronic received FDA approval for the expanded use of CRT-D in mildly-symptomatic heart failure patients. The expanded indication includes New York Heart Association (NYHA) Class II heart failure patients with a left ventricular ejection fraction (LVEF) of less than or equal to 30 percent, left bundle branch block (LBBB), and a QRS duration greater than or equal to 130 milliseconds. Nearly 200,000 Americans are considered NYHA Class II, with another 620,000 people worldwide fitting this designation.
Medtronic received FDA approval for the expanded use of CRT-D in mildly-symptomatic heart failure patients. The expanded indication includes New York Heart Association (NYHA) Class II heart failure patients with a left ventricular ejection fraction (LVEF) of less than or equal to 30 percent, left bundle branch block (LBBB), and a QRS duration greater than or equal to 130 milliseconds. Nearly 200,000 Americans are considered NYHA Class II, with another 620,000 people worldwide fitting this designation.