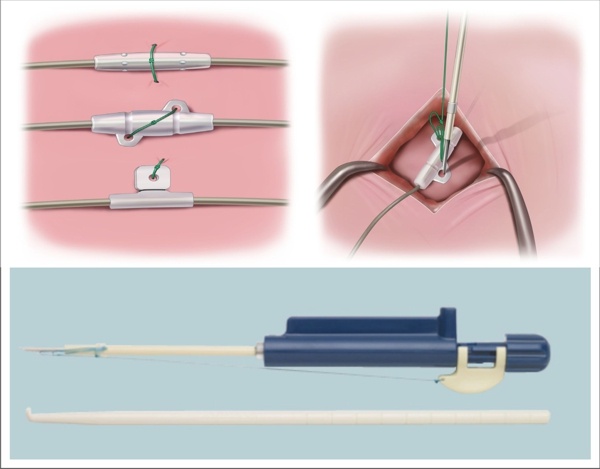
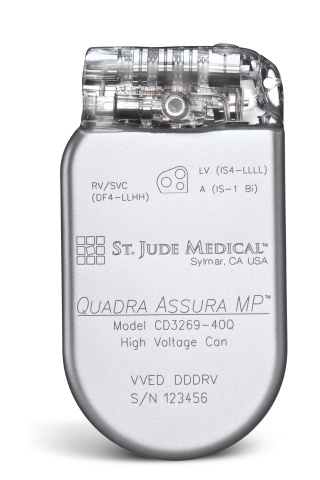
 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
According to the press release:
The Quadra Assura MP CRT-D is designed to work with the Quartet™ Lead, which has four electrodes to offer maximum flexibility for different pacing configurations. The new MPP capability allows physicians to program simultaneous or sequential delivery of two left ventricular (LV) pulses per pacing cycle, rather than the standard single pacing pulse. The capability to deliver two LV pulses per cycle allows physicians to tailor CRT pacing for each patient, potentially leading to more effective outcomes compared to single site pacing, which may be particularly beneficial in patients not responding to traditional bi-ventricular pacing therapy.

 Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:
Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:

 St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.
St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.
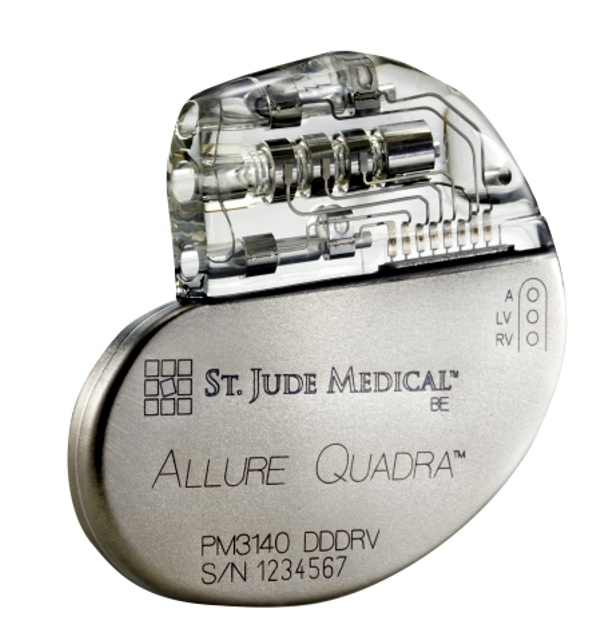 St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:
St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release: