Medtronic announced today its financial results for its first quarter of fiscal year 2020. As a whole, the company reported first quarter worldwide revenue of $7.493 billion, an increase of 1.5 percent.
However, Cardiac Rhythm & Heart Failure decreased 3.1 percent as reported (1.2 percent on a constant currency basis). According to the press release”
“Arrhythmia Management grew in the mid-single digits on a constant currency basis, driven by mid-single digit growth in Pacemakers, including mid-twenties growth of the Micra® transcatheter pacing system, as well as mid-thirties growth of the TYRX® absorbable antibacterial envelope, high-single digit growth of the Reveal LINQTM insertable cardiac monitoring system, and high-single digit growth in AF Solutions, all on a constant currency basis. Arrhythmia Management growth was offset by low-double digit declines in Heart Failure, including high-forties declines in sales of left ventricular assist devices (LVADs), both on a constant currency basis.”
Pain Therapies, the other area of Medtronic’s business that depends heavily on active implantable medical devices also took a hit, with first quarter revenue of $292 million decreased 7.0 percent as reported or 6.1 percent on a constant currency basis. The announcement explains:
“Pain Stimulation declined in the low-double digits, reflecting channel destocking and the overall slowdown of the spinal cord stimulation market.”



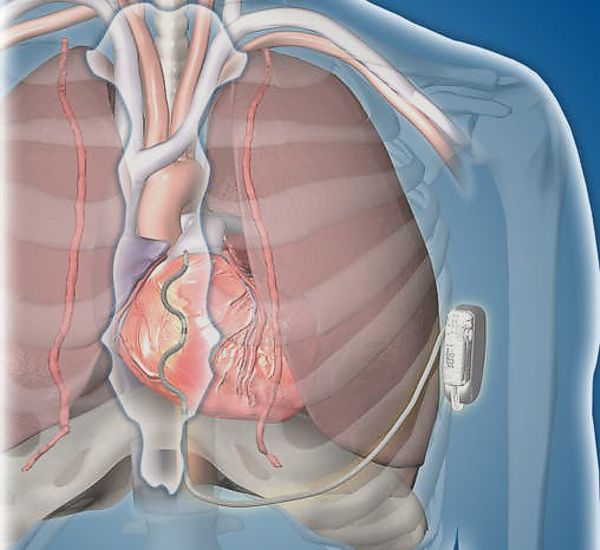



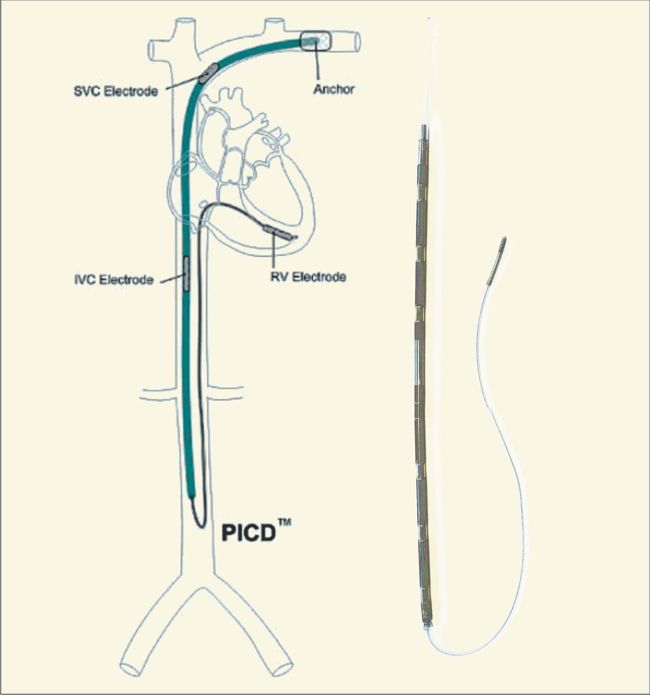
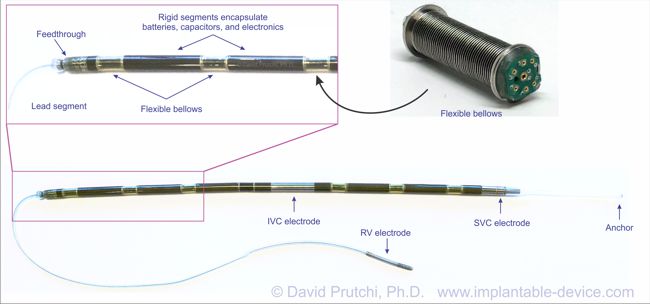
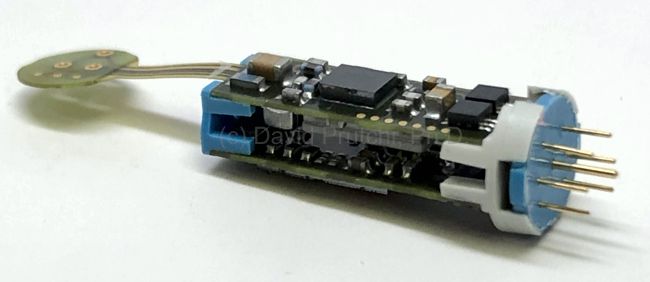
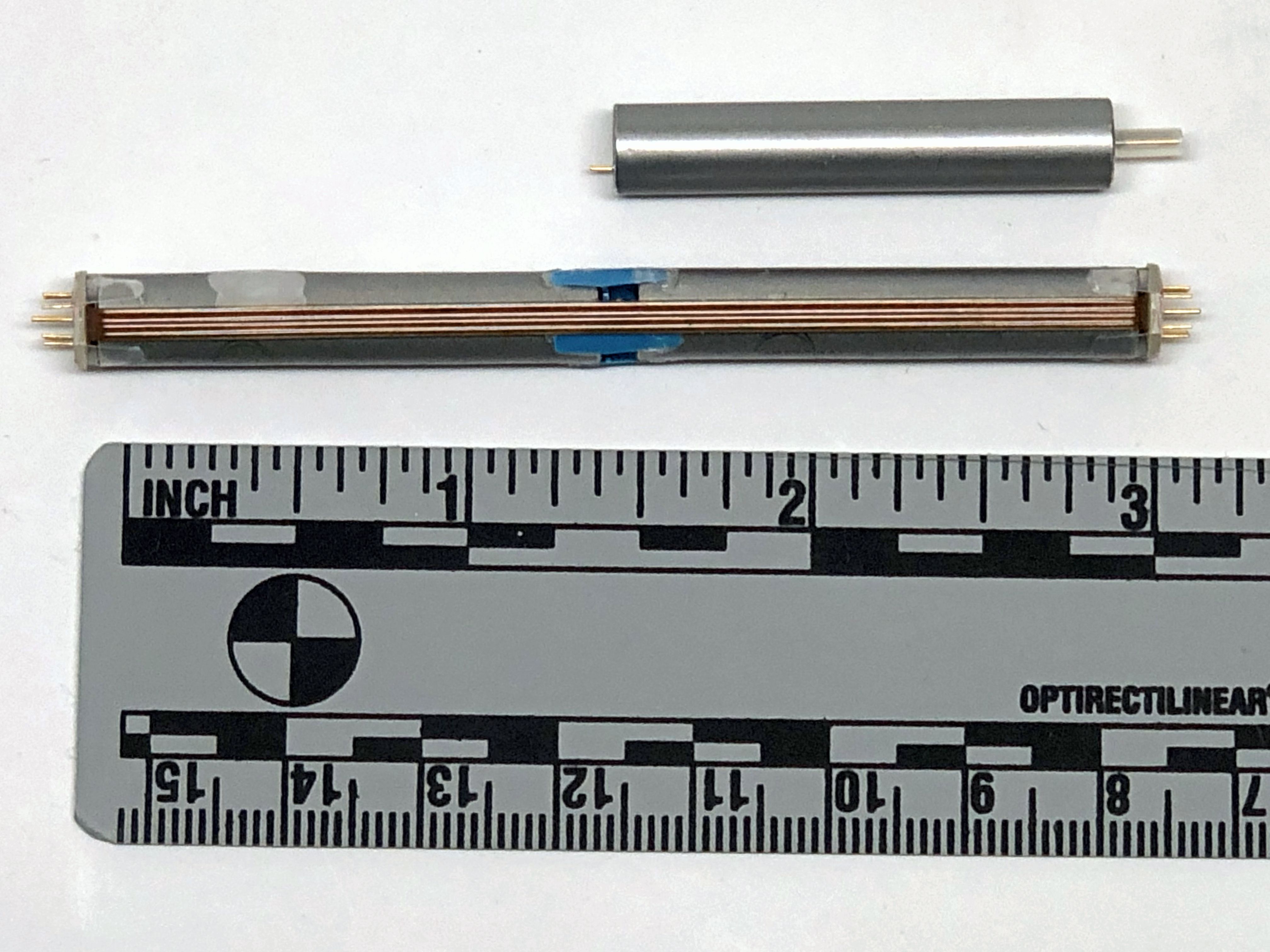
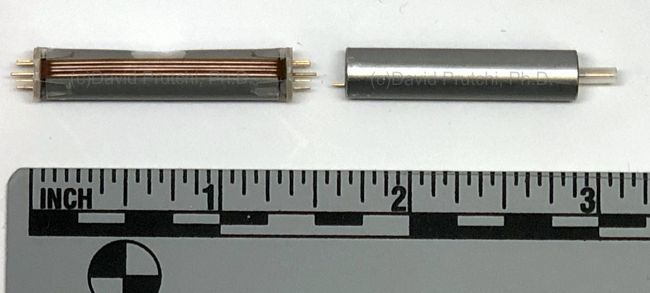




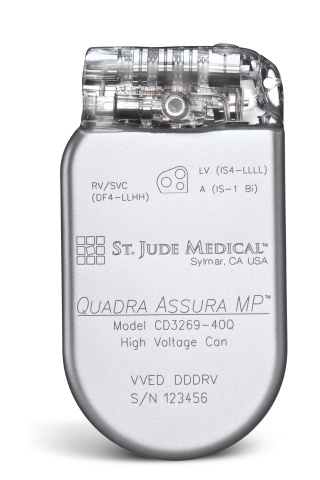 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.