Sorin announced the CE Mark approval and first implant of its KORA 100 pacing system.
According to the press release:
KORA 100 SR and DR pacemakers when implanted with the Sorin BEFLEX pacing lead enable implanted patients to undergo magnetic resonance imaging (MRI) safely.[1] Pacemaker patients are typically older than 65 years. This patient population is prone to other medical conditions such as arthritis, cancer or stroke that may require MRI examinations for optimal diagnosis and management.
The KORA 100 pacing system has been designed with both patients and physicians in mind. Device settings which allow safe operation during an MRI examination are automatically enabled when KORA 100 pacemakers detect the scanner’s magnetic field. Similarly the devices sense when a patient leaves the MRI field, and return to normal operation within five minutes. The Automatic MRI Mode feature, which is patented and available exclusively on Sorin Group pacemakers, limits the amount of time that KORA 100 pacemakers operate in MRI mode.






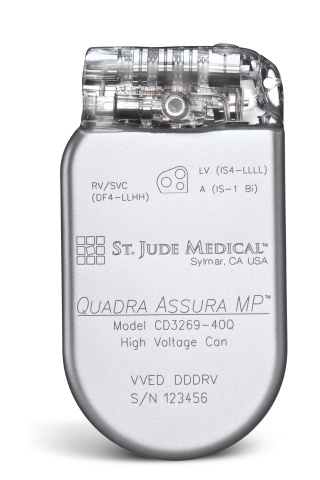 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs. Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:
Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:

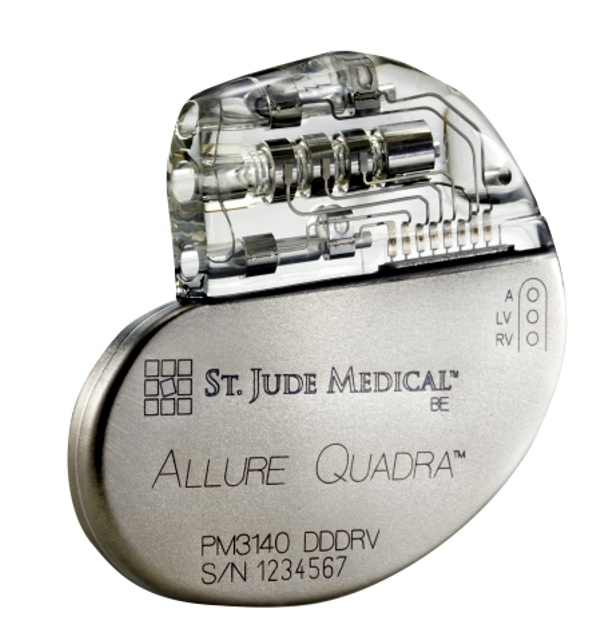 St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:
St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:
 Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.
Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.