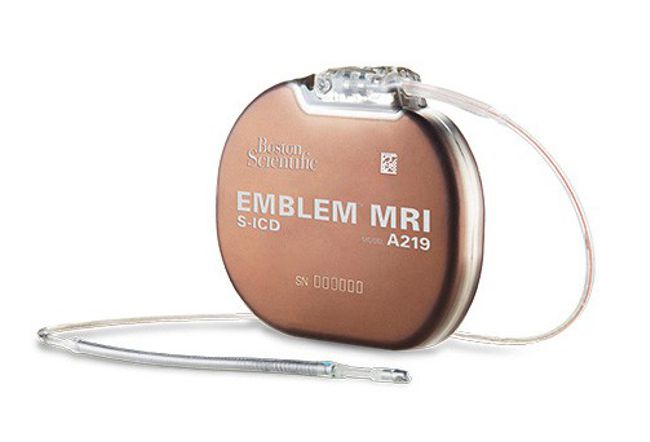
Boston Scientific presented the final results from the UNTOUCHED study of the EMBLEM™ Subcutaneous Implantable Defibrillator (S-ICD) System presented at HRS 2020 SCIENCE.
The global, prospective, non-randomized UNTOUCHED study evaluated the safety and efficacy of the EMBLEM S-ICD System for primary prevention of SCD, specifically in patients with a LVEF ≤35%.
Data demonstrated S-ICD therapy had an inappropriate shock-free rate of 95.9% at 18-months post-procedure, meeting the primary endpoint with a rate comparable to or lower than those seen in previous S-ICD and transvenous implantable cardioverter-defibrillator (TV-ICD) studies, conclusively showing high efficacy and safety of the EMBLEM S-ICD for patients without pacing indications.
Results for the investigator-sponsored, prospective, randomized, head-to-head PRAETORIAN trial using the EMBLEM S-ICD were also presented, indicating that the S-ICD can be the preferred therapy choice for the majority of ICD-indicated patients without a need for pacing as it offers comparable performance while avoiding lead-related complications and serious infections associated with TV-ICDs.

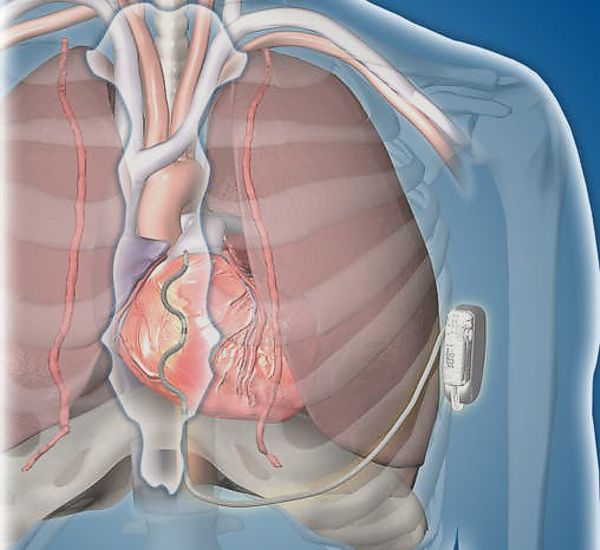








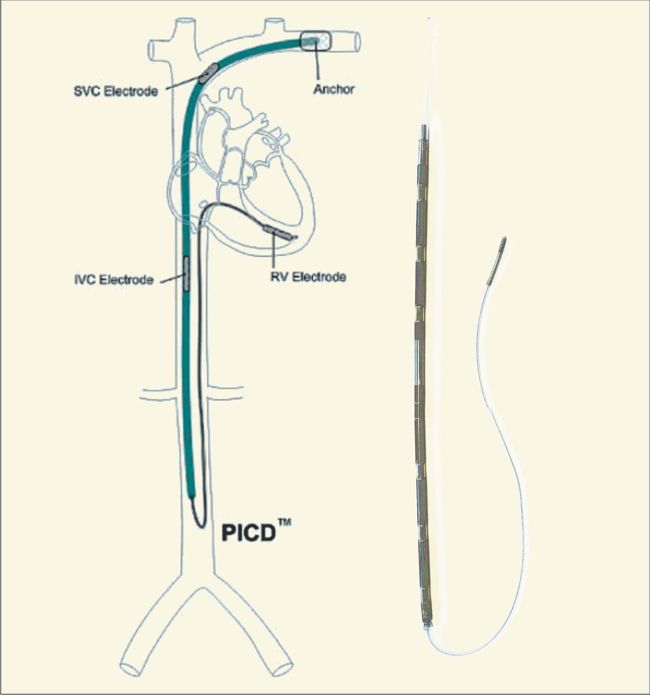
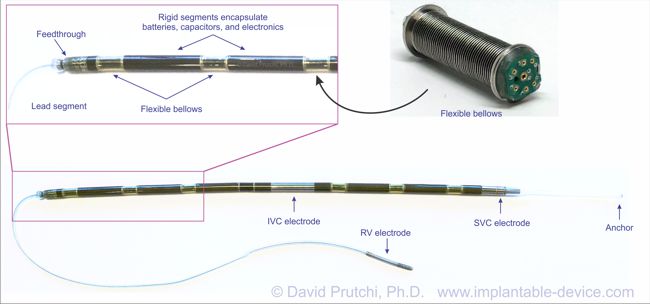
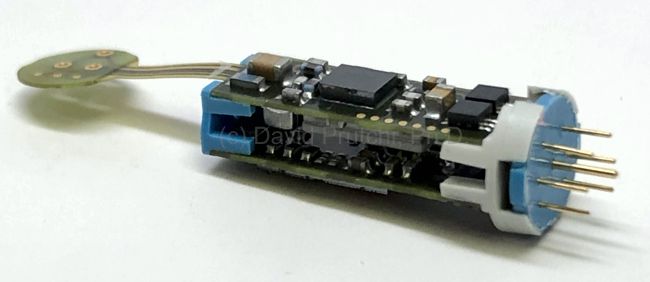
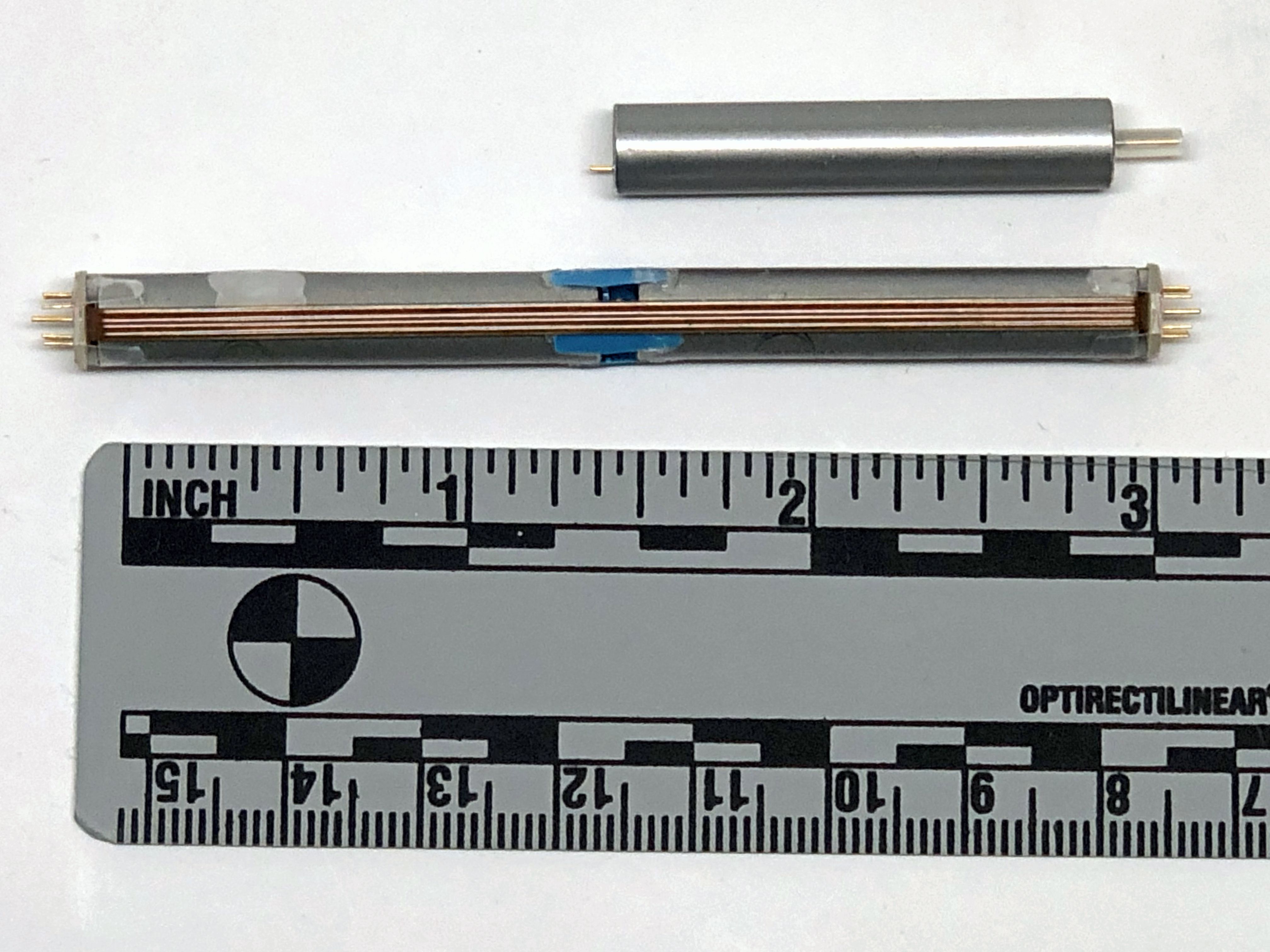
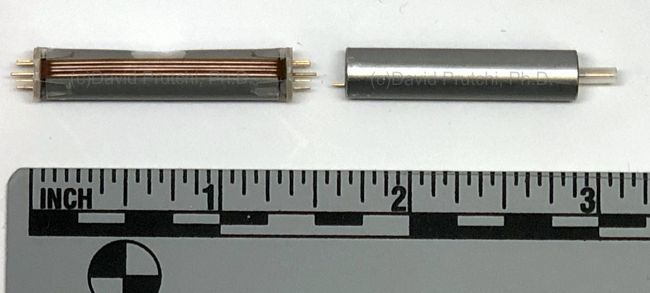


 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
