
Although the FDA advisory panel found CardioMEMS’ CHAMPION HF Pressure Measurement System to be safe, on October 9, 2013, the majority of panel members decided that the implantable sensor monitor to help guide treatment in patients with congestive heart failure is not effective.
According to Forbes, one panel member said it was difficult to connect the dots between use of the monitor and decreased hospitalization and lack of data on use in women.
This is not the first time that CardioMEMS attempts to receive PMA approval. In 2011 FDA rejected the Champion implantable monitoring system for heart failure for premarket approval, in part due to questions about the validity of presented data. Last year, CardioMEMS received an FDA Warning Letter.


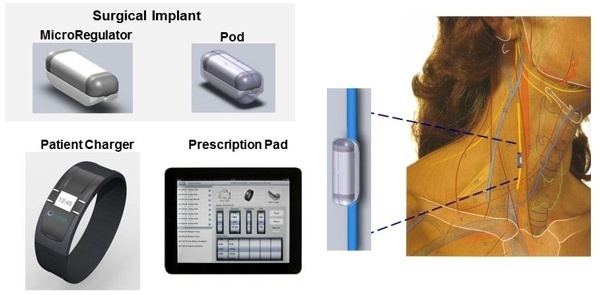
 Net product sales increased 12.4% to $67.4 million in the first fiscal quarter ended July 26 for Cyberonics, Inc. of Houston, TX. Including license revenue, sales were up 14.2% overall, with Europe in particular contributing a strong performance. Diluted earnings per share were adjusted by $0.17 cents due to a litigation settlement.
Net product sales increased 12.4% to $67.4 million in the first fiscal quarter ended July 26 for Cyberonics, Inc. of Houston, TX. Including license revenue, sales were up 14.2% overall, with Europe in particular contributing a strong performance. Diluted earnings per share were adjusted by $0.17 cents due to a litigation settlement.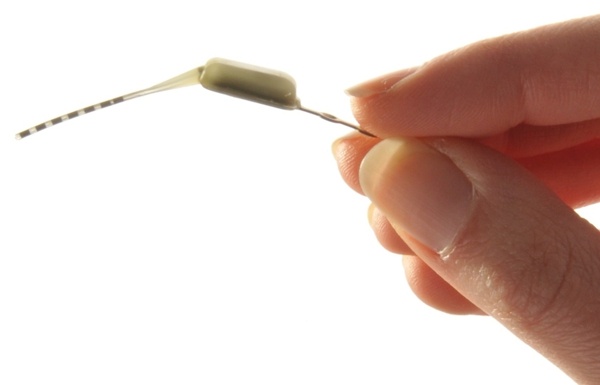
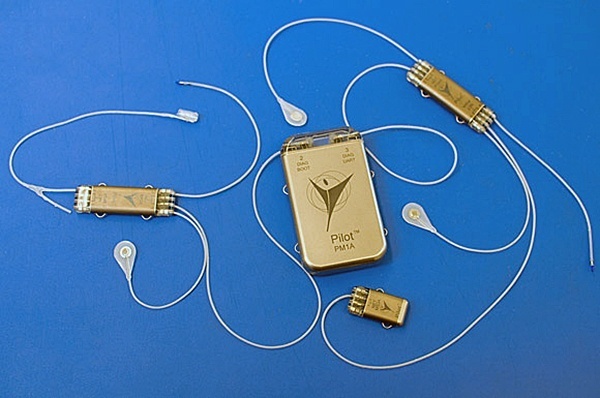
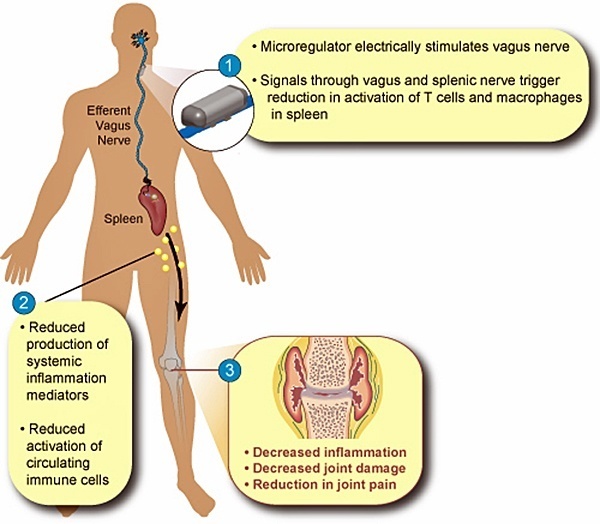

 Start-up company
Start-up company  Boston Scientific announced that it has launched a clinical trial to determine whether occipital nerve stimulation (ONS) using the Precision™ System can safely and effectively treat chronic migraine when used in conjunction with anti-migraine medications. According to the press release:
Boston Scientific announced that it has launched a clinical trial to determine whether occipital nerve stimulation (ONS) using the Precision™ System can safely and effectively treat chronic migraine when used in conjunction with anti-migraine medications. According to the press release: Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently
Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently 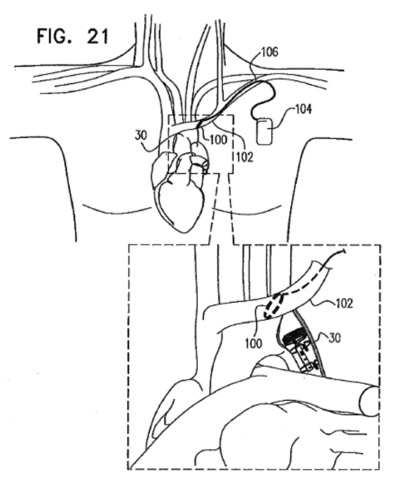
 NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.
NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.