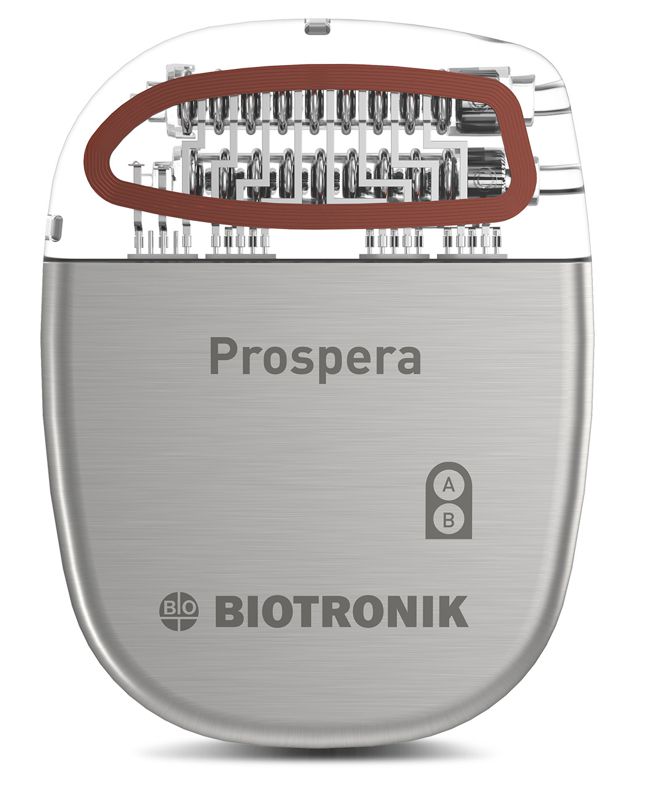
Image Credit: Biotronik
In 2023 Biotronik received FDA approval for its Prospera™ Spinal Cord Stimulation System to treat chronic, intractable pain. The system features connectivity with Biotronik’s Embrace One™, which the company claims to provide automatic, objective, remote daily data transmission for true proactive care management.
A new study using the Prospera® SCS system was published in Neuromodulation supporting the adoption of real-time remote monitoring as a new standard for long-term SCS device management.
According to the press release, the study demonstrates that:
“…automatic, daily remote monitoring of spinal cord stimulation (SCS) devices enables the first real-time visibility into post-implant therapy use, including the identification of “virtual explants” — patients with prolonged device inactivity who have not undergone surgical removal. In the study, patients using the Prospera® SCS system — the only SCS device with automatic, objective, daily remote monitoring and remote programing — maintained high rates of therapy use and low explant rates.”







 Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.
Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD. Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.