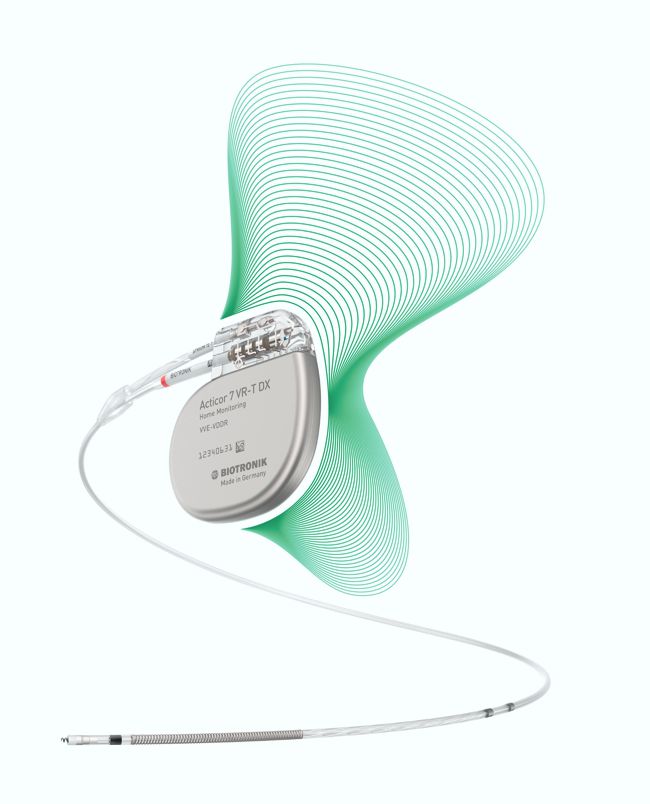
 Biotronik announced yesterday the full commercial launch of the Acticor device family, including Acticor DX and CRT-DX devices. The unique feature of the system is the single-pass lead with its floating atrial sensing rings. According to the press release:
Biotronik announced yesterday the full commercial launch of the Acticor device family, including Acticor DX and CRT-DX devices. The unique feature of the system is the single-pass lead with its floating atrial sensing rings. According to the press release:
“When implanted with Biotronik’s Plexa ProMRI S DX lead, the hybrid ICD Acticor systems offer dual-chamber diagnostics without the need for an atrial lead. Importantly, all three Acticor DX devices feature a new DF4 header configuration with a penta-polar electrode lead cable design that simplifies the implant procedure for physicians.”


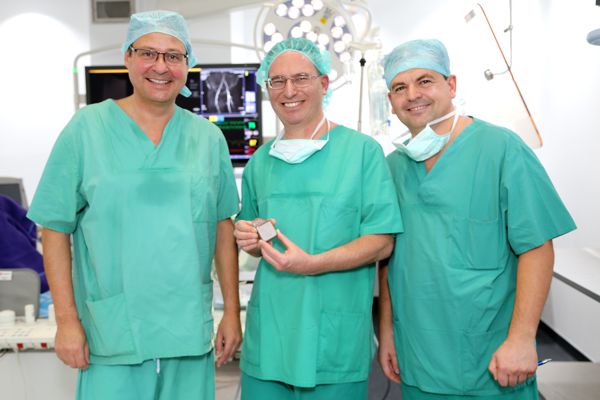
 I have led the development of devices to deliver CCM™ therapy from the very beginning, so it is with great pleasure and pride that I share with you the exciting news that Impulse Dynamics just received approval from the United States Food and Drug Administration (FDA) for our Optimizer® Smart System for heart failure patients! An official FDA announcement was made through the publication of a press release that you can find here:
I have led the development of devices to deliver CCM™ therapy from the very beginning, so it is with great pleasure and pride that I share with you the exciting news that Impulse Dynamics just received approval from the United States Food and Drug Administration (FDA) for our Optimizer® Smart System for heart failure patients! An official FDA announcement was made through the publication of a press release that you can find here: 




 A
A 

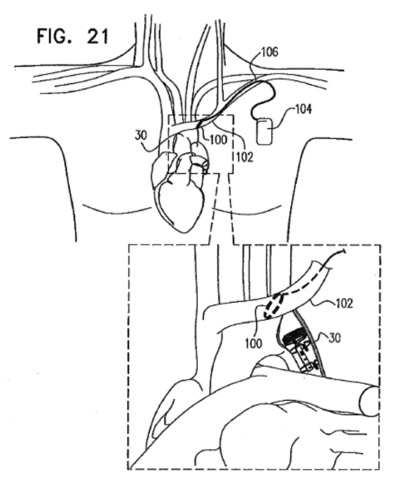
 NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.
NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.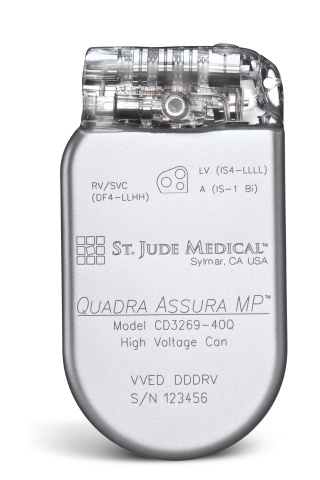 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.