
Image Credit: NeuroPace
NeuroPace of Mountain View, CA received FDA approval for MR-Conditional labeling of its RNS® implantable system for the treatment of medically refractory partial epilepsy.
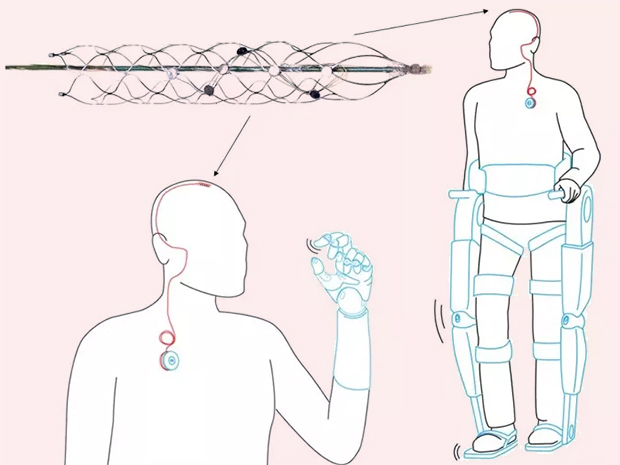
Unlike Cyberonics’ VNS IPGs, the RNS® neurostimulator is designed to detect abnormal electrical activity in the brain and respond by delivering electrical stimulation to normalize brain activity before the patient experiences seizure symptoms. The neurostimulator is implanted in the cranium and connected to one or two leads that are implanted near the patient’s seizure focus.








 I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”:
I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”:
