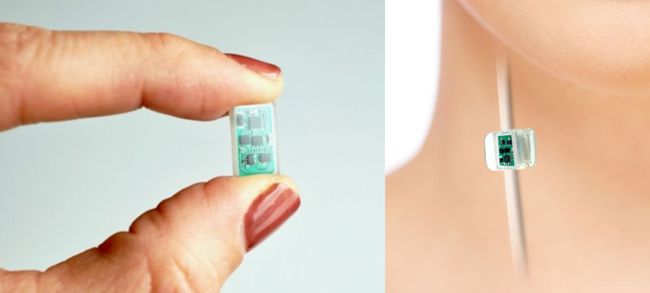
Image Credit: SetPoint Medical
On July 31, 2025, SetPoint Medical received FDA approval for its neuroimmune modulation device for the treatment of adults living with moderate-to-severe rheumatoid arthritis (RA) who are not adequately managed by—or cannot tolerate—existing advanced RA therapies, such as biological and targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs).
FDA’s approval was based on the results of the 242-patient randomized, double-blind, sham-controlled, RESET-RA study, that demonstrated the SetPoint System’s safety and efficacy in patients with moderately to severely active RA who had an incomplete response or intolerance to one or more biologic or targeted synthetic DMARDS. The study met its primary efficacy endpoint of ACR20 at three months, with improvements observed in ACR response rates and disease activity metrics through 12 months of follow-up. 75% of patients in the study were free of biologic or targeted synthetic DMARDs at 12 months.
This week SetPoint announced that it raised $140M in private financing. According to the press release, proceeds of the financing will support commercialization of the SetPoint System, as well as advancement of the company’s pipeline in other autoimmune conditions.