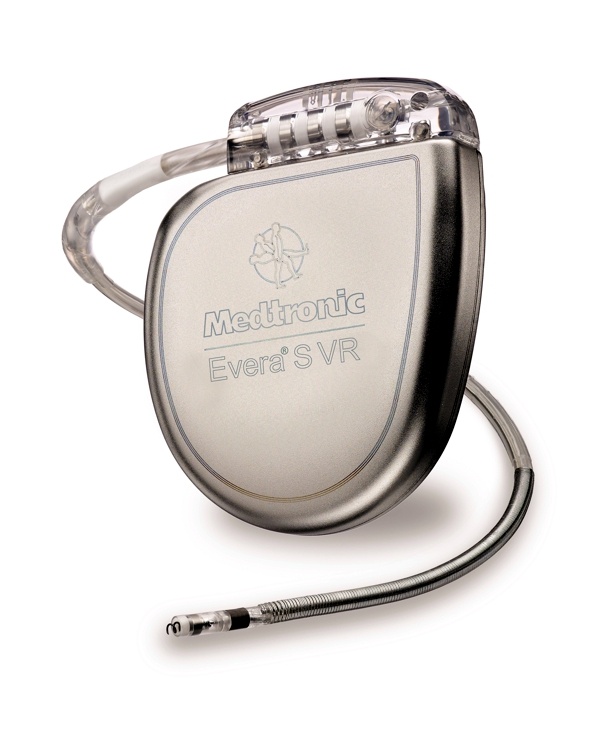
Medtronic announced today the FDA approval of its new Viva® portfolio of cardiac resynchronization therapy with defibrillation (CRT-D) devices, and the Evera® portfolio of implantable cardioverter-defibrillators (ICD). According to the press release:
“The Viva CRT-D significantly improves response rate to the therapy for many indicated heart failure patients, with a demonstrated 21 percent reduction in overall heart failure hospitalizations within the first year after implant as compared to historical CRT trials. According to economic analyses presented at ISPOR Europe, with this device both payers and hospital providers will experience reductions in overall healthcare costs as compared to CRT-D devices with traditional programming.

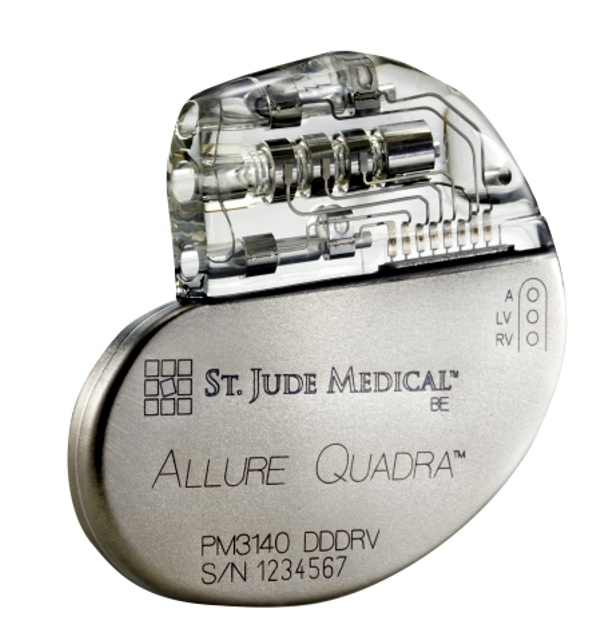 St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:
St. Jude Medical today announced CE Mark approval and European launch of its Allure Quadra™ Cardiac Resynchronization Therapy Pacemaker (CRT-P), which brings the quadripolar lead technology to the pacemaker market for the first time. According to the press release:




 Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.
Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.