On February 4, 2013, after more than 20 years of research and development, Second Sight Medical Products, Inc., announced that its Argus® II Retinal Prosthesis System (“Argus II”) has received U.S. market approval from the Food and Drug Administration (FDA) to treat individuals with late stage retinitis pigmentosa (RP). This announcement follows receipt of the European approval in 2011, and a unanimous recommendation by the FDA’s Ophthalmic Devices Advisory Panel in September 2012 that this revolutionary product be made available to treat this patient population in the U.S.
Category Archives: Therapies
AIMDs classified according to general type of therapy
Medtronic Introduces First Neuromodulation Systems Compatible with Full-Body MRI
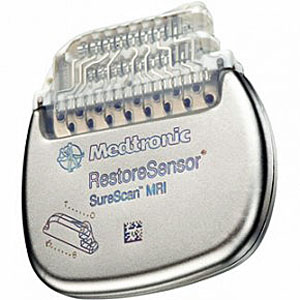
Medtronic has introduced in Europe the first and only implantable neurostimulation systems indicated for use in the treatment of chronic back and/or leg pain that are designed for full-body Magnetic Resonance Imaging (MRI) scans under specific conditions. Medtronic SureScan neurostimulation systems include enhancements to existing devices as well as specially designed leads to reduce or eliminate the hazards produced by the MRI environment. The devices also include a proprietary SureScan programming feature, which sets the device into an appropriate mode for the MRI environment.
SetPoint Medical’s Vagus Nerve Stimulation for Treatment of Systemic Inflammation
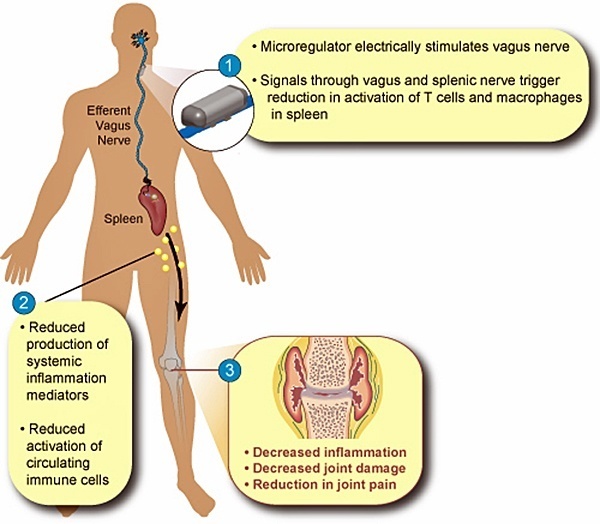
SetPoint Medical, headquartered in Valencia, California, is developing neuromodulation therapies for patients with inflammatory autoimmune diseases, such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD), psoriasis, diabetes, heart disease, and multiple sclerosis. SetPoint’s proprietary neuromodulation platform consists of an implantable “microregulator”, wireless charger and iPad prescription pad application.
Functional Neuromodulation Ltd. Starts Study using DBS of the Fornix (DBS-f) for Mild Alzheimer’s
 Toronto-based Functional Neuromodulation announced that it implanted the first U.S. Alzheimer’s patient in the “ADvance Study” with a deep brain stimulation (DBS) system meant to improve cognitive performance. ADvance will evaluate the safety and potential clinical benefit of DBS of the fornix (DBS-f), a major inflow and output pathway in the brain’s memory circuit, for patients with mild Alzheimer’s. The ADvance Study is being conducted using Medtronic Activa DBS IPGs.
Toronto-based Functional Neuromodulation announced that it implanted the first U.S. Alzheimer’s patient in the “ADvance Study” with a deep brain stimulation (DBS) system meant to improve cognitive performance. ADvance will evaluate the safety and potential clinical benefit of DBS of the fornix (DBS-f), a major inflow and output pathway in the brain’s memory circuit, for patients with mild Alzheimer’s. The ADvance Study is being conducted using Medtronic Activa DBS IPGs.
While DBS has been an effective treatment for movement disorders for more than 15 years, it was only recently that this approach was first applied to Alzheimer’s. Dr. Andres Lozano, a neurosurgeon at University of Toronto and Scientific Founder of Functional Neuromodulation, originated the concept of treating memory disorders using deep brain stimulation (DBS) while treating a patient suffering from morbid obesity. In this patient, DBS stimulation of the hypothalamus and fornix was associated with an unexpected observed improvement in the patient’s memory.
Boston Scientific’s Precision Spectra™ SCS with 32 Contacts and 32 Dedicated Power Sources Receives CE Mark

Boston Scientific Received European Regulatory Approval For New Precision Spectra™ Spinal Cord Stimulator System. It is the first and so-far only SCS system with 32 contacts and 32 dedicated power sources designed to provide pain relief to a broad spectrum of chronic pain patients.
Boston Scientific Defibrillators Receive CE Mark for 10-Year Longevity Projections

Image Credit: Boston Scientific
Boston Scientific has received CE Mark approval for increased longevity projections for the INCEPTA™, ENERGEN™, PUNCTUA™, COGNIS® and TELIGEN® implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-Ds). The longevity projections are based on data submitted to the European authorities and vary for each device dependent on the model type and settings.
Projected device longevity exceeds 10 years for some models, approaches eight years for its CRT-D devices, and according to the press release is up to double that of comparable competitive device models. The company supports these devices with warranties of up to 10 years:
Warranties: INCEPTA and ENERGEN VR ICD: 10 years; INCEPTA and ENERGEN DR ICD: eight years; PUNCTUA and TELIGEN ICD: seven years; INCEPTA and ENERGEN CRT-D: six years; and PUNCTUA & COGNIS CRT-D: five years.
Biotronik Launches Implantable BioMonitor for Monitoring Arrhythmias
 Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
According to Biotronik’s press release:
“BioMonitor® is a subcutaneous implantable leadless cardiac monitor for the long-term continuous remote monitoring of patients with arrhythmias such as AF, bradycardia, sudden rate drop, asystole and tachycardia.
As sensitivity and specificity are essential in the detection of arrhythmias such as AF, BIOTRONIK has developed ClearSense Technology with a unique three-vector signal detection that produces highly precise and reliable arrhythmia monitoring.
Boston Scientific Receives CE Mark for RELIANCE® 4-FRONT™ Defibrillation Lead

Image Credit: Boston Scientific
Boston Scientific has received regulatory approval to market the RELIANCE® 4-FRONT™ lead, its next generation implantable defibrillation lead now available in Europe and Asia. According to the press release, “The RELIANCE 4-FRONT lead is built upon the demonstrated performance and reliability of the RELIANCE lead platform with design enhancements intended to simplify the implant procedure. With more than 350,000 implants worldwide, RELIANCE leads have demonstrated a 98.9 percent survival probability at 8 years.
“Leveraging the RELIANCE platform, we made a series of targeted design enhancements with RELIANCE 4-FRONT to improve and simplify implantation,” said Kenneth Stein, M.D., chief medical officer of Boston Scientific’s Cardiac Rhythm Management Group. “RELIANCE 4-FRONT represents our continued dedication to product innovation to meet the needs of patients and physicians.”
Boston Scientific Receives CE Mark of Vercise™ Deep Brain Stimulation System
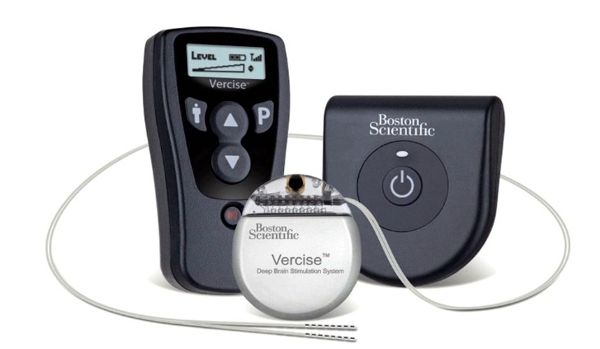
Image Credit: Boston Scientific
Boston Scientific Corporation received CE Mark approval for use of its Vercise™ Deep Brain Stimulation (DBS) System for the treatment of Parkinson’s disease. The Vercise DBS System is the first and only commercially available DBS system to incorporate multiple independent current control, which is designed to selectively stimulate targeted areas in the brain. This system is an innovative technology that is designed to provide physicians fine control of stimulation.
First Human Use of 24-Electrode Retinal Implant by Bionic Vision Australia
Bionic Vision Australia researchers announced on August 30, 2012 that they successfully performed the first implantation of an early prototype bionic eye with 24 electrodes.
According to the press release:
One for History Buffs: Pacemaker Made in Brasil

My friend Daniel Villamil from CCC Medical in Uruguay sent me these pictures of a very unique device in his colection. It is a late-1960s/early 1970s pacemaker made in Sao Paulo, Brasil.
UPDATE Oct 3, 2012:
CCC’s CEO Julio Arzuaga recalled that this pacemaker was manufactured in the early 1960s by the Instituto de Cardiologia Dante Pazzanese in Sao Paulo, Brasil. The physicians leading the pacemaker team were Dr. Decio Kormann and Dr. Adib Jatene.
Dr. Orestes Fiandra used to implant these Brasilian pacemakers in Uruguay. However, they were not very reliable. For this reason, and with help from Drs. Kormann and Jatene, Dr. Fiandra started CCC del Uruguay as a more industrial environment for the production of pacemakers.

St. Jude Medical Receives CE Mark Approval of Eon Mini to Treat Chronic Migraine
 St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.
St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.
According to the press release:
“Intractable chronic migraine is one of the most difficult-to-treat headache disorders,” said Professor Gennaro Bussone, M.D., head of the Neurological Department at Istituto Besta in Milan Italy. “By definition, people living with this condition are spending half their month living with debilitating headaches. This therapy expands our options in helping manage patients who suffer with disabling chronic migraine symptoms.”
Boston Scientific Receives FDA Approval of S-ICD® for Patients at Risk of SCA
On Sept. 28, 2012 Boston Scientific announced that the FDA granted regulatory approval for its S-ICD® System, the world’s first and only commercially available subcutaneous implantable defibrillator (S-ICD) for the treatment of patients at risk for sudden cardiac arrest (SCA). The S-ICD System sits entirely just below the skin without the need for leads to be placed into the heart.
Human Implants of Vestibular Prostheses at the Maastricht University Medical Center
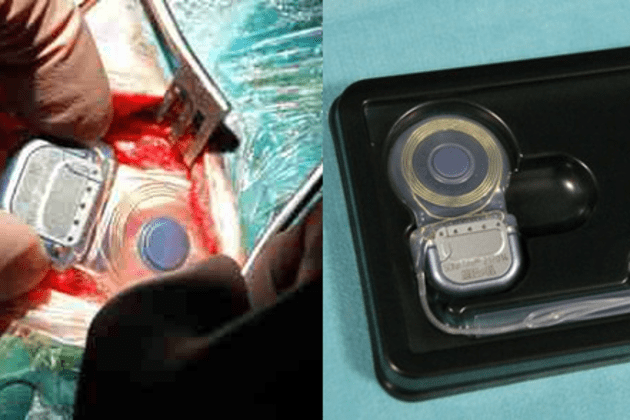
Physicians at the Maastricht University Medical Center implanted two patients with vestibular prostheses. These implants are similar to a cochlear implant, but instead of sound, conveys orientation and acceleration information to the vestibular organ that assists in preserving balance.
According to lead implanter Prof. Robert Stokroos, preliminary assessment is promising, and research will be conducted to determine if the brain can integrate the information from these implants to restore balance to the patients.
There have been a number of prior human implants of different vestibular devices. For example, in 2010 Jean-Philippe Guyot implanted a vestibular nerve stimulation device in a human subject. Their work was published in March 2011. The device was a modified Med-El cochlear implant using an electrode that was implanted near the left posterior ampullary nerve. In addition, Washington University implanted a device based on a similar approach (a modified Nucleus device by Cochlear Ltd.) on Oct. 21, 2010. It must be noted however that these prior devices do not sense head motion, but were designed to override abnormal vestibular signals. On the other hand, from the sketchy details that have appeared, it seems that the devices implanted in Maastricht are the first true vestibular prostheses capable of responding to orientation and acceleration.
TEDxCambridge Talk Calls for Open Data from AIMDs
In this TED talk, Hugo Campos explains his frustration with the fact that his ICD collects data, but he – as a patient – is unable to access these data as a diagnostic tool to help make good choices about eating, exercise and other activities.


