 CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.
CCC is one of the oldest pacemaker manufacturers in the world. It was founded in 1969 by Dr. Orestes Fiandra, who performed the first succesful, human, long-term pacemaker implant in the world.This was achieved in Uruguay on February 2, 1960 by Dr. Orestes Fiandra and Dr. Roberto Rubio. The pacemaker was manufactured by Dr. Rune Elmqvist of Elema-Schönander in Sweden, and was implanted in Uruguay in a 34-year-old patient with AV block. This unit worked successfully for nine and a half months, until the patient died of sepsis from an unrelated infection. Continue reading


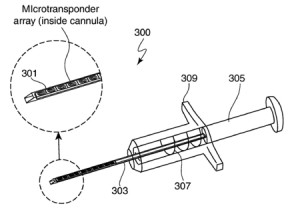

 Intrapace was founded in Mountain View, CA by
Intrapace was founded in Mountain View, CA by  Transneuronix, Inc. was founded in 1995 and was based in Mount Arlington, New Jersey. It was acquired by Medtronic in 2005.
Transneuronix, Inc. was founded in 1995 and was based in Mount Arlington, New Jersey. It was acquired by Medtronic in 2005.
 Transoma was the name that Data Sciences International of St Paul, MN adopted in 2003 when it re-fucused its animal telemetry implant business to develop an implantable wireless system to capture electrocardiogram data for diagnosing human cardiac arrhythmias, as well as to monitor the electrical activity of the heart and transmit data from the patient’s home to monitoring centers.
Transoma was the name that Data Sciences International of St Paul, MN adopted in 2003 when it re-fucused its animal telemetry implant business to develop an implantable wireless system to capture electrocardiogram data for diagnosing human cardiac arrhythmias, as well as to monitor the electrical activity of the heart and transmit data from the patient’s home to monitoring centers.

