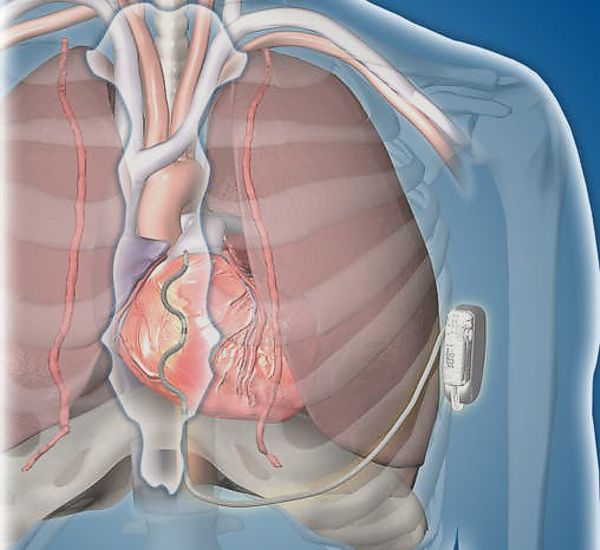
Image Credit: CVRx
Minneapolis-based CVRx received FDA approval yesterday for its BAROSTIM NEO™ device to treat Heart Failure.
The BAROSTIM NEO™ implantable pulse generator is implanted below the collar bone and is connected to a lead that attaches to the carotid artery in the neck, targeting its baroreceptors. The proposed mechanism of action is that the activation of baroreceptors cause the brain to relax the blood vessels and inhibit the production of stress-related hormones to reduce heart failure symptoms.
The no-panel approval was based on results from CVRx’s BeAT-HF phase III trial on 264 patients randomized to two arms: 130 subjects randomized to receive a BAROSTIM NEO™ were compared to 134 subjects randomized to stay on guideline-directed medical therapy. The implanted group showed the following improvement in symptomatic endpoints as compared to the control group:
- Improved their MLWHFQ score by 14 points,
- Improved their 6-minute hall walk by 60 meters, and
- Improved their NYHA status.
According to FDA’s press release, the BAROSTIM NEO™ System is indicated for the improvement of symptoms in patients with advanced heart failure (EF≤35%) who are not suited for treatment with other heart failure devices.
CVRx website: www.cvrx.com






 Impulse Dynamics – the company for which I work –
Impulse Dynamics – the company for which I work –