
Image Credit: St. Jude Medical
St. Jude today announced FDA approval of its Ellipse™ ICD. The device’s shape was designed with feedback from more than 200 physicians from around the world. The Ellipse ICD offers physicians unique design advancements, resulting in a high-energy ICD that occupies barely 30cc.
According to the announcement, “The Ellipse ICD’s unique shape was conceptualized by physicians during focus groups where they crafted in clay their vision for the ideal device design. The physician-inspired shape is unlike any device currently available and designed to increase patient comfort and physician ease-of-use. The angled header and rounded edges were designed to improve the way a lead wraps around the device once connected, which can result in a smaller incision and reduced pocket size for the device.”



 Yesterday Boston Scientific announced financial results for the first quarter ended March 31, 2012. Sales of Cardiac Rhythm Management devices were $501M vs. $559M for Q1 a year ago, or a decrease of 10%. Sales of Neuromodulation devices increased by 8% a year ago from $77M to $84M for Q1.
Yesterday Boston Scientific announced financial results for the first quarter ended March 31, 2012. Sales of Cardiac Rhythm Management devices were $501M vs. $559M for Q1 a year ago, or a decrease of 10%. Sales of Neuromodulation devices increased by 8% a year ago from $77M to $84M for Q1. St. Jude Medical today
St. Jude Medical today 

 Medtronic received FDA approval for the expanded use of CRT-D in mildly-symptomatic heart failure patients. The expanded indication includes New York Heart Association (NYHA) Class II heart failure patients with a left ventricular ejection fraction (LVEF) of less than or equal to 30 percent, left bundle branch block (LBBB), and a QRS duration greater than or equal to 130 milliseconds. Nearly 200,000 Americans are considered NYHA Class II, with another 620,000 people worldwide fitting this designation.
Medtronic received FDA approval for the expanded use of CRT-D in mildly-symptomatic heart failure patients. The expanded indication includes New York Heart Association (NYHA) Class II heart failure patients with a left ventricular ejection fraction (LVEF) of less than or equal to 30 percent, left bundle branch block (LBBB), and a QRS duration greater than or equal to 130 milliseconds. Nearly 200,000 Americans are considered NYHA Class II, with another 620,000 people worldwide fitting this designation.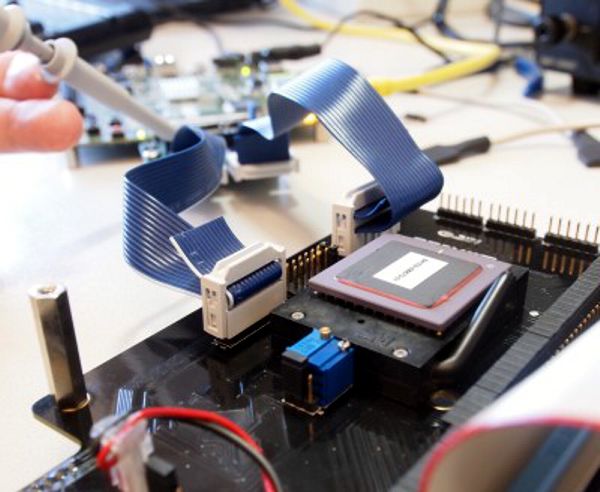
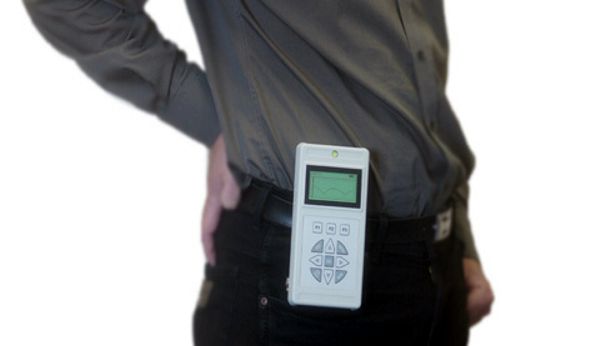
 Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.
Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.


