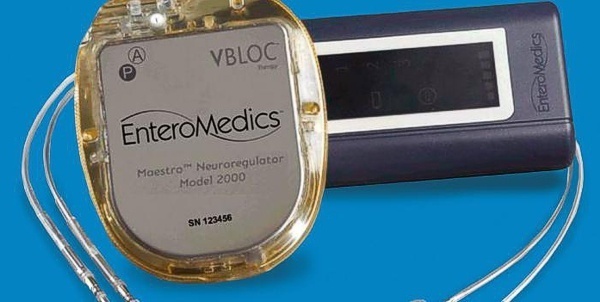
EnteroMedics reported new clinical trial data for its Maestro system, which is designed to treat obesity through vagus nerve stimulation. The company said that its Maestro RC system lost 25% of their excess weight, or 10% of their total body weight, after 18 months. Patients who received a sham implant lost 12% of excess weight, or 4% of their total weight. The company is running a five-year study of the system. The system is approved in Europe and Australia, but not the U.S.




 St. Jude announced today that it has initiated a clinical study of the Prodigy™ neurostimulator, which is the first SCS system able to deliver a proprietary mode of stimulation therapy called burst stimulation.
St. Jude announced today that it has initiated a clinical study of the Prodigy™ neurostimulator, which is the first SCS system able to deliver a proprietary mode of stimulation therapy called burst stimulation.
 A
A