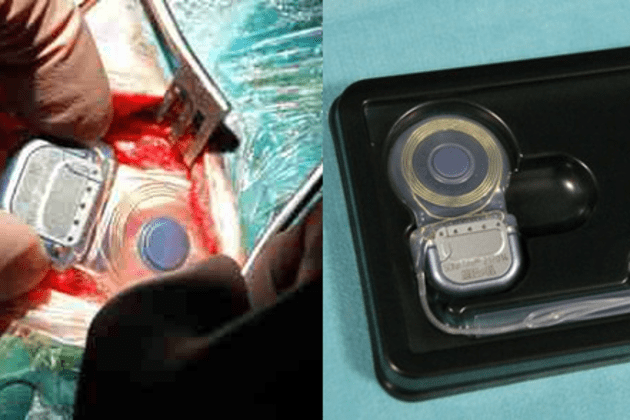
Today Boston Scientific reported its results for Q3 2012. For its AIMD divisions:
Cardiac Rhythm Management sales dropped from $503M in Q3 of 2011 to $462M for this year (-8%), a 6% decrease on constant-currency basis.
Neuromodulation sales grew by 5% from $84M to $88M.

 Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
Biotronik announced the European market release of BioMonitor®, an implantable cardiac device designed for the highly accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.

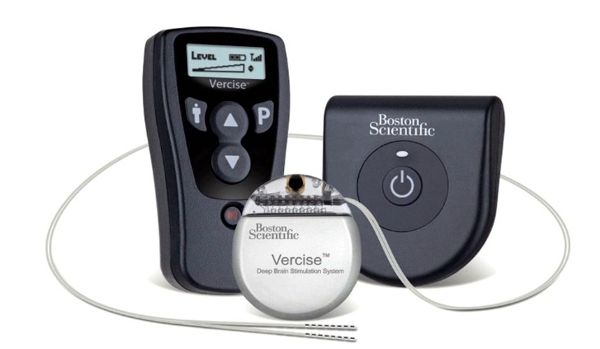


 St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.
St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.