Image Credit: Resonetics
Resonetics was founded in 1987 as a provider of engineering and manufacturing solutions for the life sciences industry. Traditionally, they offered laser processing, centerless grinding, nitinol processing, thin-wall stainless steel & precious metal tubing, photochemical machining, microfluidics manufacturing, and sensor technology.
In 2022, they acquired EaglePicher’s medical battery division, expanding on the components that it can provide to the AIMD industry.
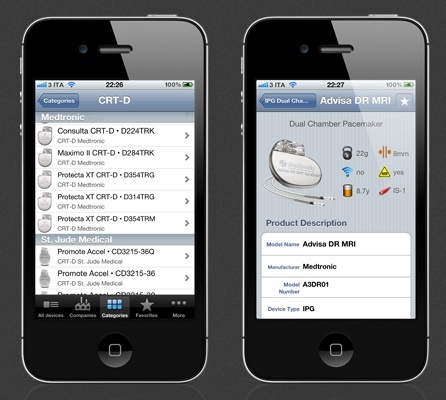
Yesterday, Resonetics announced that it has acquired the manufacturing assets, process know-how, and intellectual property related to implantable and external pulse generators (IPG/EPG) and leads manufacturing and assembly from Med-Ally, a medical technology design and development company focused on the neuromodulation market and based in Charleston, South Carolina. This integration enables Resonetics to offer customers the development of complete AIMDs
As part of the deal, the acquired assets will ultimately be transferred to an existing Resonetics facility.


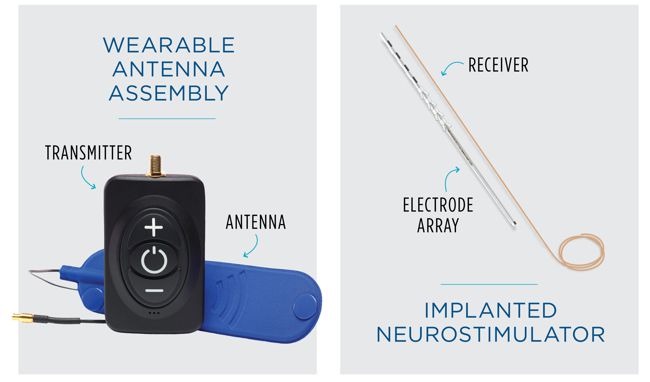
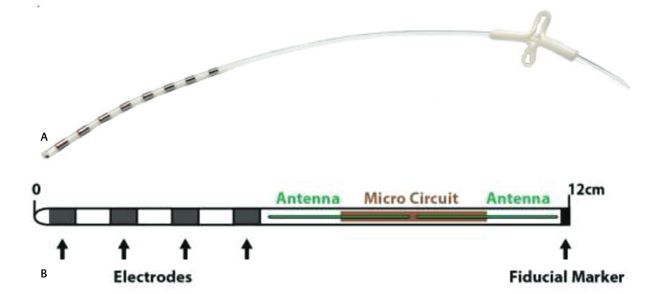

 FDA has published a draft of the guidance document that it has developed to assist industry by identifying issues related to cybersecurity that manufacturers should consider in preparing premarket submissions for medical devices. This guidance document is intended to supplement FDA’s “Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices” and “Guidance to Industry: Cybersecurity for Networked Medical Devices Containing Off-the-Shelf (OTS) Software”
FDA has published a draft of the guidance document that it has developed to assist industry by identifying issues related to cybersecurity that manufacturers should consider in preparing premarket submissions for medical devices. This guidance document is intended to supplement FDA’s “Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices” and “Guidance to Industry: Cybersecurity for Networked Medical Devices Containing Off-the-Shelf (OTS) Software”
 Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.
Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.