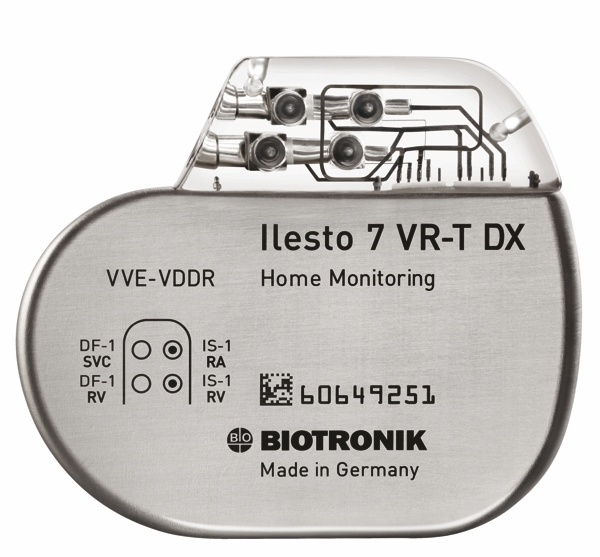
 Biotronik announced that FDA granted approval for its Ilesto 7 implantable cardioverter-defibrillator/cardiac resynchronization therapy defibrillator (ICD/CRT-D) series. According to the press release:
Biotronik announced that FDA granted approval for its Ilesto 7 implantable cardioverter-defibrillator/cardiac resynchronization therapy defibrillator (ICD/CRT-D) series. According to the press release:
“BIOTRONIK lives up to its reputation for excellence in design and manufacturing with the introduction of the Ilesto family, and the new Ilesto DX device. Physicians depend on complete and timely information, and Ilesto DX with BIOTRONIK Home Monitoring® certainly delivers. With this device, physicians can receive atrial information to ensure diagnostic accuracy and identify previously undetected atrial fibrillation. They also receive peace of mind that there is less risk of complications due to the single lead,” said Paul Woodstock, executive vice president of sales and marketing at BIOTRONIK, Inc., USA. “Ilesto’s smaller footprint will be more comfortable as well, which may present a win-win solution for patients and physicians alike.”

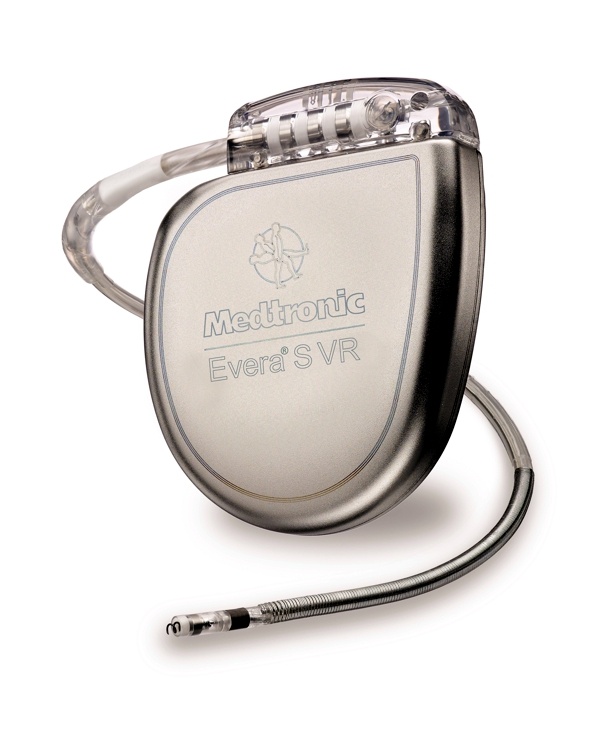

 Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.
Biotronik announced that it had received CE-approval for the world’s first DF4 ICD/CRT-D series approved for MRI. In addition, this series contains one of the world’s smallest ICDs– the Iforia single chamber ICD.





 InControl was founded in 1990 in Redmond, WA to develop an implantable device for treating atrial fibrillation. In November 1995, InControl announced the first human implant of its Metrix atrioverter.
InControl was founded in 1990 in Redmond, WA to develop an implantable device for treating atrial fibrillation. In November 1995, InControl announced the first human implant of its Metrix atrioverter. Cameron Health was founded in 2000 in San Clemente, CA to develop a leadless implantable defibrillator.
Cameron Health was founded in 2000 in San Clemente, CA to develop a leadless implantable defibrillator.