
St. Jude announced today that it has launched the SENSE™ Subcutaneous and Epidural Neuromodulation System Evaluation study – a clinical study to evaluate the combination of peripheral nerve field stimulation (PNfS), and spinal cord stimulation (SCS), to determine whether the two therapies together offer more effective management of chronic low back and leg pain than SCS alone.
According to the press release:
Peripheral nerve field stimulation and SCS are minimally-invasive neurostimulation therapies that involve the implant of a stimulation device and small electrical leads. In SCS, leads are placed in the epidural space to interrupt or mask the transmission of pain signals to the brain. In PNfS, leads are placed just under the skin in the subcutaneous tissue to stimulate the network of peripheral nerve fibers in order to reduce the pain at the location where it is most severe. PNfS is not the same as peripheral nerve stimulation (PNS), which targets a specific nerve. Instead, PNfS targets a more general network of nerves.

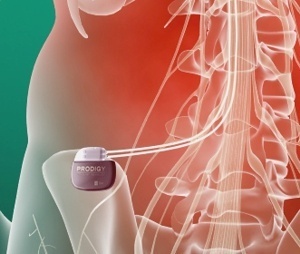 St. Jude announced today that it has initiated a clinical study of the Prodigy™ neurostimulator, which is the first SCS system able to deliver a proprietary mode of stimulation therapy called burst stimulation.
St. Jude announced today that it has initiated a clinical study of the Prodigy™ neurostimulator, which is the first SCS system able to deliver a proprietary mode of stimulation therapy called burst stimulation.
 A
A 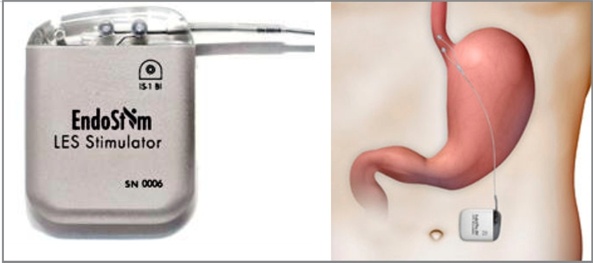









 Neuros Medical received an Investigational Device Exemption to conduct a pivotal clinical trial to evaluate the Altius™ System High Frequency Nerve Block technology for the management of intractable limb pain of amputees. The prospective, randomized, controlled pivotal clinical trial will consist of 130 patients at 15 institutions in the U.S. to evaluate the safety and efficacy of Neuros Medical’s Altius System.
Neuros Medical received an Investigational Device Exemption to conduct a pivotal clinical trial to evaluate the Altius™ System High Frequency Nerve Block technology for the management of intractable limb pain of amputees. The prospective, randomized, controlled pivotal clinical trial will consist of 130 patients at 15 institutions in the U.S. to evaluate the safety and efficacy of Neuros Medical’s Altius System.