Miniature, ultra-flexible electrode (credit: EPFL)
Aleva Neurotherapeutics is a spinoff of the Swiss Federal Institute of Technology (EPFL) Microsystems Laboratory. Aleva is developing unique microfabricated devices to more specifically target deep-brain stimulation.
kurtzweilai.net recently published an interesting blog about the technology. From the post:
“Miniature, ultra-flexible electrodes could be the answer to more successful treatment for Parkinson’s diseases, according to Professor Philippe Renaud of the École Polytechnique Fédérale de Lausanne (EPFL) in Switzerland.
He has developed soft arrays of miniature electrodes in his Microsystems Laboratory that open new possibilities for more accurate and local deep brain stimulation (DBS).

![logo_mainstay1[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/logo_mainstay11.jpg)
![SynchroMed-II[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/SynchroMed-II1-300x272.jpg)
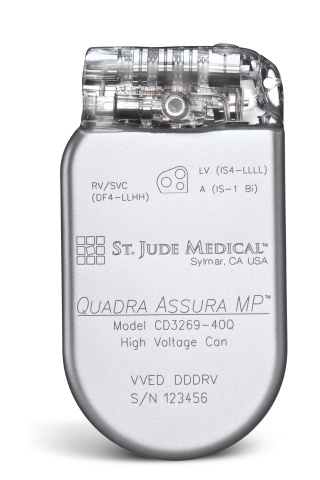 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
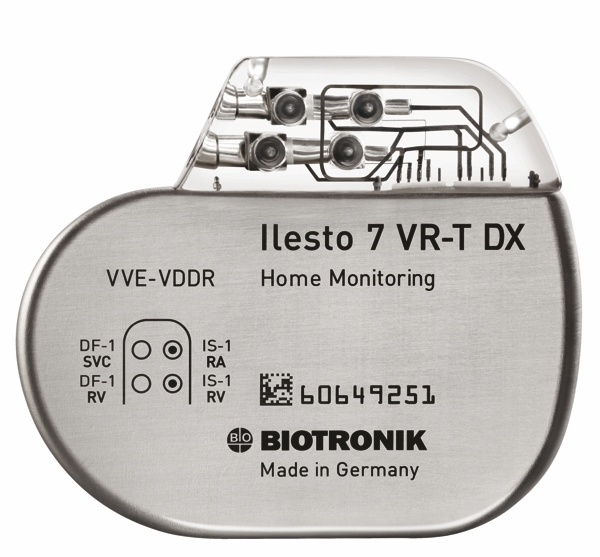
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs. Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:
Sorin announced today CE mark approval and the European commercial launch of the REPLY ™ 200 family of pacemakers featuring Sleep Apnea Monitoring (SAM). According to the press release:
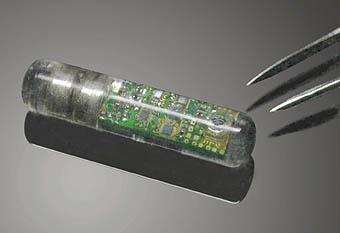

 FDA has published a draft of the guidance document that it has developed to assist industry by identifying issues related to cybersecurity that manufacturers should consider in preparing premarket submissions for medical devices. This guidance document is intended to supplement FDA’s “Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices” and “Guidance to Industry: Cybersecurity for Networked Medical Devices Containing Off-the-Shelf (OTS) Software”
FDA has published a draft of the guidance document that it has developed to assist industry by identifying issues related to cybersecurity that manufacturers should consider in preparing premarket submissions for medical devices. This guidance document is intended to supplement FDA’s “Guidance for the Content of Premarket Submissions for Software Contained in Medical Devices” and “Guidance to Industry: Cybersecurity for Networked Medical Devices Containing Off-the-Shelf (OTS) Software”

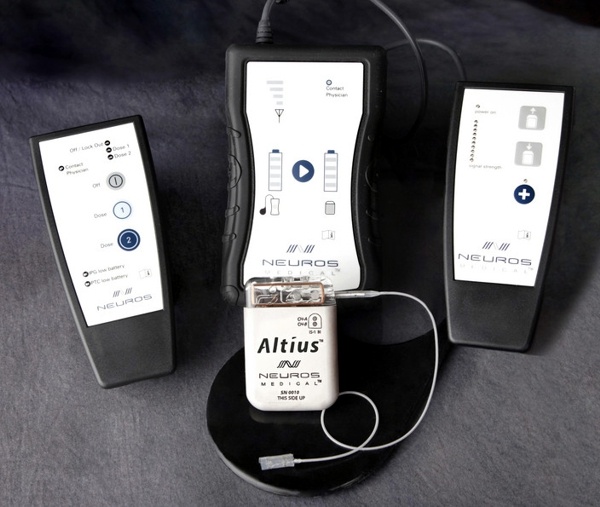
 St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.
St. Jude Medical and privately-held Spinal Modulation, Inc., today announced that they have entered into a series of agreements under which St. Jude Medical made a $40 million equity investment in Spinal Modulation, a company that has developed an innovative neuromodulation therapy that provides a new pain management option for patients with chronic, intractable pain.