 Sensors for Medicine and Science, Inc. (SMSI) of Germantown, MD was founded in 1997 to develop chemical sensing technologies based on fluorescence sensing.
Sensors for Medicine and Science, Inc. (SMSI) of Germantown, MD was founded in 1997 to develop chemical sensing technologies based on fluorescence sensing.
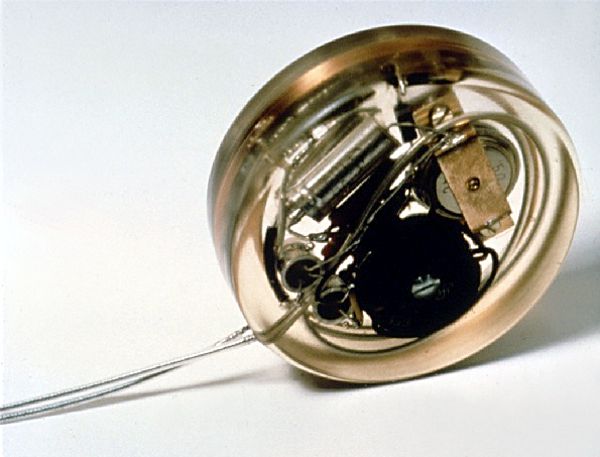
SMSI® is now developing an implantable glucose sensor that is designed to automatically measure interstitial glucose every few minutes. The sensor implant communicates wirelessly with a small external reader, allowing it to track the rate of change of glucose levels and warn the user of impending hypo- or hyperglycemia. According to SMSI, the target operational life of the sensor implant will be 6-12 months, after which it would be replaced. Continue reading













 I apologize for yesterday’s interruption of service in this blog. I moved hosting company because the old one was too slow and unreliable.
I apologize for yesterday’s interruption of service in this blog. I moved hosting company because the old one was too slow and unreliable.