
Image Credit: VeriMed
VeriMed’s VeriChip is the only RFID tag that has been cleared by FDA for human implant. The concept behind the medical use of the VeriChip is that patients would have the tiny chip implanted just under the skin, in the back of the arm. Each VeriMed microchip contains a unique identification number that emergency personnel may scan to immediately identify the patient and access his/her personal health information, thus facilitating appropriate treatment without delay. This is especially important for patients who suffer from conditions that may render them unconscious, confused, or unable to communicate. Although the FDA approved the use of the device for anyone 12 years of age or older, it would mostly be recommended for patients with diabetes, stroke, seizure disorders, dementia, Alzheimer’s, developmental disorders, and organ transplants.



 Leptos Biomedical was founded in Fridley, MN in 2002 by Dr. John D. Dobak. Leptos intended to develop an implantable device to stimulate the greater splanchnic nerve, that was hoped would result in reduced food intake and increased energy expenditure.
Leptos Biomedical was founded in Fridley, MN in 2002 by Dr. John D. Dobak. Leptos intended to develop an implantable device to stimulate the greater splanchnic nerve, that was hoped would result in reduced food intake and increased energy expenditure.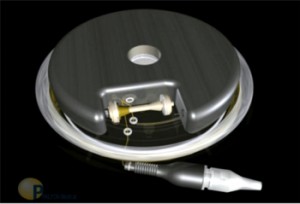

 We conduct reliability analyses for our implantable devices on a continued basis. I’ve spent the last few days readying the data for this period’s analysis, and thought that a short primer on how this is actually done would be of interest to fellow engineers who may need it at some point.
We conduct reliability analyses for our implantable devices on a continued basis. I’ve spent the last few days readying the data for this period’s analysis, and thought that a short primer on how this is actually done would be of interest to fellow engineers who may need it at some point.

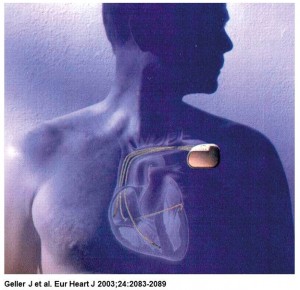 InControl was founded in 1990 in Redmond, WA to develop an implantable device for treating atrial fibrillation. In November 1995, InControl announced the first human implant of its Metrix atrioverter.
InControl was founded in 1990 in Redmond, WA to develop an implantable device for treating atrial fibrillation. In November 1995, InControl announced the first human implant of its Metrix atrioverter.



