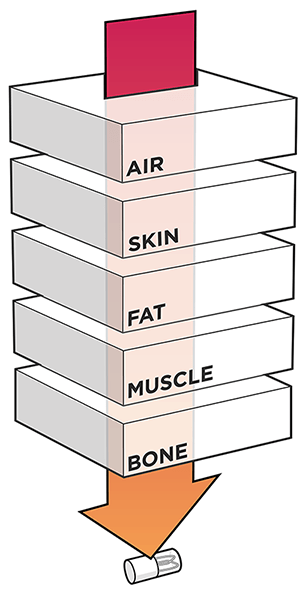
Neuspera’s Mid-Field Powering technology. Image credit: Neuspera
Neuspera is a startup company located in San Jose, CA. They are developing miniaturized neuromodulation implants that are externally powered from a wearable device.
According to Neuspera, their “Mid-Field Powering” technology uses evanescent and propagating electromagnetic waves to power implanted medical devices to beyond 10cm of depth. Their technology is claimed to use the body as a natural waveguide to focus power ensuring energy is delivered to where it is needed.
In addition to coming up with their own neuromodulation system, Neuspera hopes to license its Mid-Field Powering technology to recharge or power other types of implantable devices. According to their technical publications and patent, they use a phased array microwave transmitter operating at a 1.6GHz local minimum in tissue absorption to maximize power transfer. Per the patent, if the power coupled into tissue is allowed to meet the maximum permitted level of exposure, up to 2.2 mW can be transferred by their system.
Neuspera Medical announced that it has raised a new total of $26 million in equity financing, following the closing of the second tranche of its series B round, to help fund clinical testing programs for its neuromodulation implants.
A vide explaining Neuspera’s technology is available at:
Neuspera Medical from Tiffany Wise on Vimeo.
Neuspera’s website is: http://neuspera.com

















 I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”:
I can’t remember exactly where I found the picture of a Pacesetter model BD102 VVI, but the story behind it is documented by Kirk Jeffrey in “Machines in our Hearts”: