
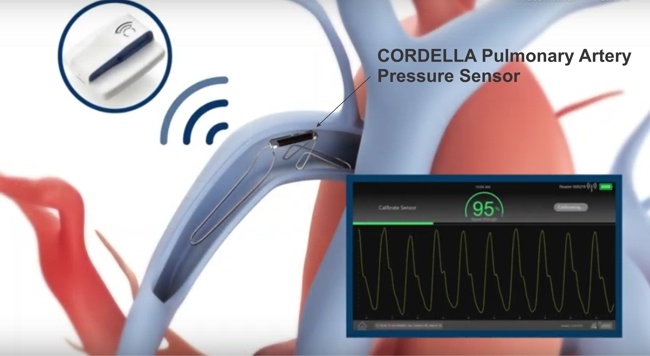
Image Credit: Valencia Technologies
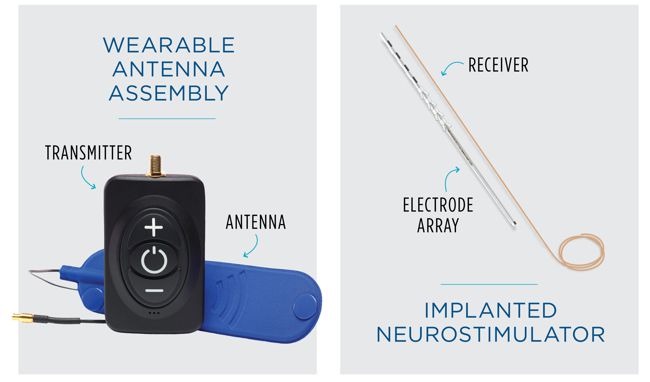
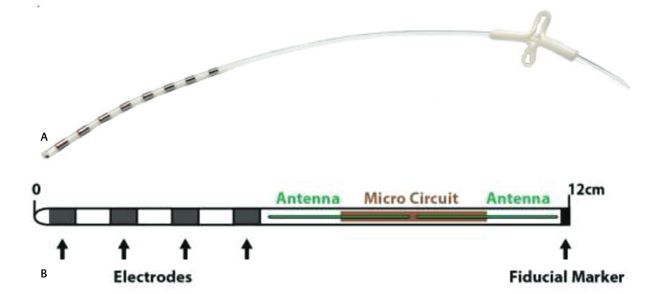
On March 1, 2022 FDA approved Valencia Technologies’ eCoin Peripheral Neurostimulator System to stimulate the tibial nerve to treat Urge Urinary Incontinence. The eCoin system delivers electrical pulses to the tibial nerve in 30-minute sessions based on a fixed schedule. A healthcare provider can adjust the level of stimulation based on each patient’s sensitivity and needs, using the remote control.
Boston Scientific today announced it has entered into a definitive agreement to acquire Valencia Technologies Corporation. Terms of the acquisition have not been disclosed.









 Impulse Dynamics, the company where I’m CTO and Executive VP,
Impulse Dynamics, the company where I’m CTO and Executive VP, 