 Today Boston Scientific announced its Q2 2013 results. CRM sales were 3% down from $488M for Q2 2012 to $475M this year (2% on constant-currency basis).
Today Boston Scientific announced its Q2 2013 results. CRM sales were 3% down from $488M for Q2 2012 to $475M this year (2% on constant-currency basis).
Neuromodulation went up by a whopping 21% from last year’s Q2 $91M to $111 in Q2 2013. The press release indicated that Boston Scientific achieved global year-over-year revenue growth of 21 percent in Neuromodulation on the strength of the Precision Spectra™ Spinal Cord Stimulator (SCS) System launch.

 Boston Scientific announced that it has launched a clinical trial to determine whether occipital nerve stimulation (ONS) using the Precision™ System can safely and effectively treat chronic migraine when used in conjunction with anti-migraine medications. According to the press release:
Boston Scientific announced that it has launched a clinical trial to determine whether occipital nerve stimulation (ONS) using the Precision™ System can safely and effectively treat chronic migraine when used in conjunction with anti-migraine medications. According to the press release:
 Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently
Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently 
 NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.
NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.



![logo_mainstay1[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/logo_mainstay11.jpg)
![SynchroMed-II[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/SynchroMed-II1-300x272.jpg)
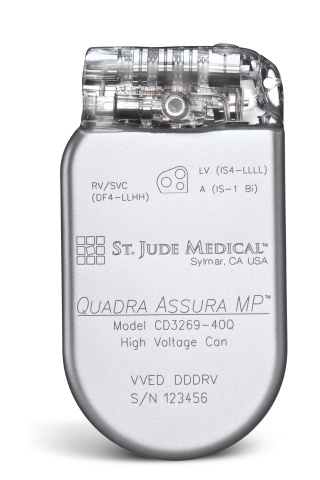 St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.
St. Jude Medical today announced CE Mark approval of its next-generation quadripolar device, the Quadra Assura MP™ cardiac resynchronization therapy defibrillator (CRT-D). The device features MultiPoint™ Pacing (MPP) technology that enables physicians to pace multiple locations on the left side of the heart. This gives the clinician more choices to best optimize cardiac resynchronization therapy (CRT) pacing to meet individual patient needs.