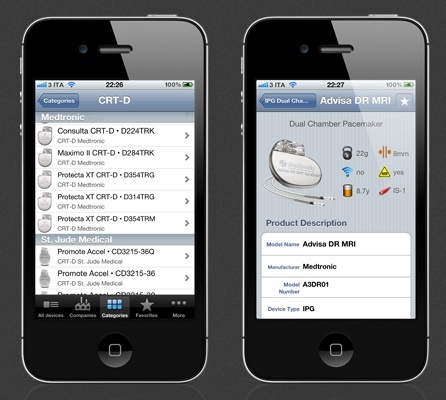
My friend and colleague Dr. Irit Yaniv alerted me to this iPhone app that was just released. It is an implantable pacemaker and defibrillator database that, according to its author, displays up to 70 parameters for each model, includes battery and longevity data, and links directly to product manuals. Continue reading
Category Archives: Bradycardia
Implantable devices for the treatment of bradycardia (pacemakers)
The Australian Pacemaker: Telectronics (1965-1995)
 In 1965, Australian medical device pioneer Noel Gray established Telectronics – Australia’s first manufacturing facility for producing pacemakers that were designed in-house. Telectronics was an innovative developer, achieving some major successes in the early cardiac pacing field, for example, Telectronics’ leads allowed narrowing the pacing pulse to its current nominal of 0.5 milliseconds; encapsulating the pacemaker in titanium instead of epoxy; using a microplasma weld to join the two halves of the pacemaker capsule; creating one of the first rate-responsive ‘demand’ pacemakers; and isolating the pacemaker’s battery in a separate compartment to deal with the problem of leaking mercury-zinc batteries. Continue reading
In 1965, Australian medical device pioneer Noel Gray established Telectronics – Australia’s first manufacturing facility for producing pacemakers that were designed in-house. Telectronics was an innovative developer, achieving some major successes in the early cardiac pacing field, for example, Telectronics’ leads allowed narrowing the pacing pulse to its current nominal of 0.5 milliseconds; encapsulating the pacemaker in titanium instead of epoxy; using a microplasma weld to join the two halves of the pacemaker capsule; creating one of the first rate-responsive ‘demand’ pacemakers; and isolating the pacemaker’s battery in a separate compartment to deal with the problem of leaking mercury-zinc batteries. Continue reading
Elema-Schoenander and the Very First Human Implants of a Pacemaker in Sweden (1958) and Uruguay (1960)
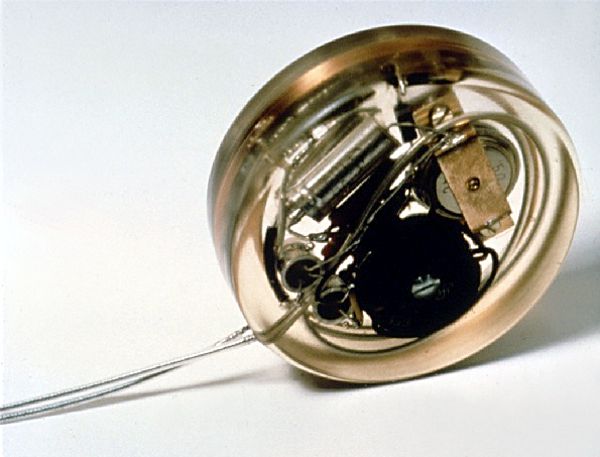
This is a picture of the first pacemaker to be implanted in a human patient. It was developed by Dr. Rune Elmqvist (1906–1996), a physician by training, but working for the Swedish company Elema-Schonander as an engineer. Dr. Elmqvist developed the device in cooperation of Åke Senning, senior physician and cardiac surgeon at the Karolinska University Hospital in Solna, Sweden. Continue reading
Intermedics’ First Pacemakers (Mid 1970s)

In 1973, former Medtronic sales representative Albert Beutel founded Intermedics in Freeport, TX. The first product was a small, mercury-cell-powered pacemaker. In 1974 Intermedics introduced a lithium-powered version, and in 1976 it introduced InterLith which was hermetically sealed, and weighed just 65 grams. At the time, InterLith’s size was a breakthrough, and became a very popular device, solidifying Intermedics’ position in the industry.
Original Datasheet for Arco’s Nuclear Pacemakers (ca. 1974)

Some time ago, my friend and colleague Paul Spehr gave me a copy of Arco Medical’s product catalog. I scanned the original datasheets for Arco Medical’s nuclear fixed-rate and demand pacemakers models NU-5 and NU-6 and posted them here in pdf format: Arco_Nuclear_Datasheets
Click here for a color picture and more information on Arco Medical’s nuclear pacemakers.
American Optical Cardio-Care II Demand Pacemaker (ca. 1971)

The Cardio Care II Pacemaker was American Optical’s second implantable device. It was an improved version of the Cardio Care pacemaker. Besides improvements to the circuitry, the circuit board was enclosed separately inside a hermetic can within the epoxy encapsulation. Continue reading
American Optical Cardio-Care Demand Pacemaker (1968)
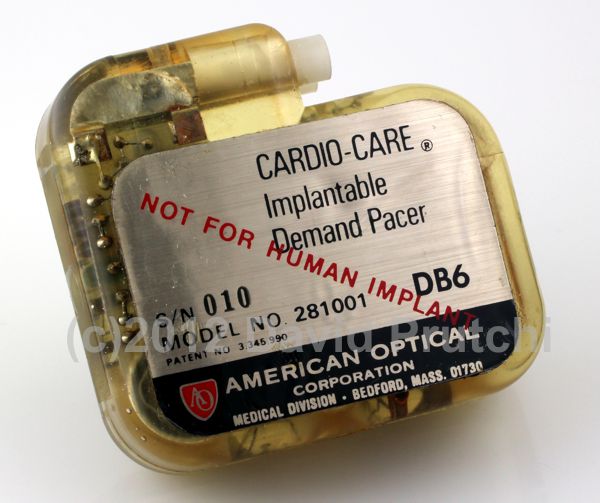
Barouh Berkovits at American Optical Co of Boston, MA designed the first “Demand Pacemaker” – what we now know as a VVI pacemaker. The Cardio-Care Demand Pacemaker, introduced in 1968, was American Optical’s first implantable device.
From Kirk Jeffrey’s Machines in our Hearts(2001):
“Berkovits in 1963 designed a sensing capability into the pacemaker. His invention behaved exactly like an asynchronous pacer until it detected a naturally occurring R wave, the indication of a ventricular contraction. This event would reset the timing circuit of the pacemaker, and the countdown to the next stimulus would begin anew. Thus the pacer stimulated the heart only when the ventricles failed to contract. It worked only ‘‘on demand.’’ As an added benefit, noncompetitive pacing extended the life of the battery. Continue reading
A Challenge to History Buffs: Who Was Digikon?

I took this picture a very long time ago at the office of one of my implanter friends in Europe. Ever since then, I’ve tried to find out about “Digikon,” but have had no luck so far. All that I have been able to find from the St. Jude legacy device database is that Digikon had produced a number of pacemaker models, including the one shown in this picture.
Do you know anything about Digikon? Please let me know!
Cook Pacemaker’s Sensor Kelvin 504 Central-Venous-Temperature-Sensing Pacemaker (ca. 1992)

In 1983, Bill Cook and Dr. Neal Fearnot began to work under the Cook Pacemaker Company in Leechburg, PA on developing the technology developed by Dr. Fearnot at Purdue University into an improved prototype for a temperature-based exercise responsive pacemaker that was released in 1988 as the Kelvin Sensor rate-responsive pacemaker. One of the first CVT rate-adaptive pacemakers was the Cook Model Kelvin 500 series. Continue reading
Intermedics’ Circadia Central Venous Temperature-Sensing Pacemaker (ca. 1993)

The Circadia pacemaker was one of the very few devices that had a lead-borne thermistor to measure cental venous temperature (CVT) as a sensor for rate-response.
A unique feature of this pacemaker was an iridium-oxide (IrOx)-coated button welded to the can. It was believed that this button would improve unipolar IEGM sensing and reduce unipolar pacing thresholds (it didn’t). Continue reading
Cook’s Sensor Kelvin and Intermedics’ Circadia Temperature-Sensing Rate-Responsive Pacemakers
 One of the indicators of metabolic demand that has been used for controlling the rate of pacemakers is central venous blood temperature (CVT).
One of the indicators of metabolic demand that has been used for controlling the rate of pacemakers is central venous blood temperature (CVT).
In 1983, Bill Cook and Dr. Neal Fearnot began to work under the Cook Pacemaker Company on developing the technology developed by Dr. Fearnot at Purdue University into an improved prototype for a temperature-based exercise responsive pacemaker that was released in 1988 as the Kelvin Sensor rate-responsive pacemaker. One of the first CVT rate-adaptive pacemakers was the Cook Model Kelvin 500 series.
Another one of the first CVT rate-adaptive pacemakers was the Intermedics Nova MR, which differs from the Kelvin 500 series in that its pacing algorithm had a more dynamic HR response. Continue reading
Medcor Corporation’s Pacemakers (ca. 1975)
 Medcor was established in Hollywood, FL in 1969, and began developing pacemakers, lead and accessories in 1971. By 1975 it had a series of lithium-powered pacemaker in the market, but they never became popular with physicians.
Medcor was established in Hollywood, FL in 1969, and began developing pacemakers, lead and accessories in 1971. By 1975 it had a series of lithium-powered pacemaker in the market, but they never became popular with physicians.
On July 1980, Daig Corporation of Minnetonka, MN acquired Medcor with the expectation that Medcor pacemaker technology could be profitably marketed.
Daig had hoped to market a new line of Medcor pacemakers, but significant electronic malfunctions were encountered which foreclosed further development Medcor’s pacemaker product line. As a result of malfunctions in the first pacemaker line, FDA approval was withheld on a second Medcor line of pacemakers due to similarities in product design.
In 1981, amidst a very serious financial condition, Daig closed its doors. The remaining assets were acquired by Daig’s largest customer – Pacesetter (now part of St. Jude Medical).
UPDATE, May 20, 2013:
Mark Christensen – who worked at Daig Corporation from 1980 to 1986 – sent me a kind note with some additional information: “As you note in your comments on Medcor, it was purchased by Daig in 1980 but remained as a separate business. Daig’s financial crisis in 1981 caused Daig to go into Chapter 11 Bankruptcy and Medcor went to Chapter 7 – liquidation and was sold at auction in late 1981. No pacemaker company bought any of the assets. Daig reorganized, diversified into EP catheters and was bought by St Jude Medical in 1996 (15 years later).” Thanks Mark!
Medtronic’s Leadless Pacemakers
Medtronic announced at TEDMED 2010 that it is working on leadless pacemakers. Dr. Stephen Osterle, senior vice president of medicine and technology and member of Medtronic’s Executive Management Team, unveiled the device. Osterle said that physicians will be able to control the device with a smart phone.
EBR System’s Wireless Pacemaker

EBR Systems, Inc., founded in 2003 and headquartered in Sunnyvale, CA, is developing the WiCS® Wireless Cardiac Stimulation technology to eliminate cardiac pacing leads, historically a major source of complications and reliability issues. The startup was spun out of research by founder Debra Echt, a former professor of medicine and a cardiologist at Vanderbilt University. Continue reading
Nanostim’s Leadless Pacemaker
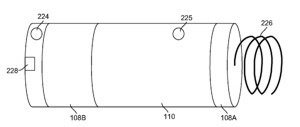 Nanostim is an early-stage AIMD company in Milpitas, CA that is developing a pacemaker that can be implanted inside the heart through a catheter. The tiny device is attached directly to the heart, eliminating the need for leads.
Nanostim is an early-stage AIMD company in Milpitas, CA that is developing a pacemaker that can be implanted inside the heart through a catheter. The tiny device is attached directly to the heart, eliminating the need for leads.
In May 2011 Nanostim announced that St. Jude Medical had made a substantial investment in the company.
The company is operating in stealth mode, but some details about the leadless pacemaker have emerged from Nanostim’s patents and patent applications. An interesting detail is about the possible use of a betavoltaic power source: Continue reading