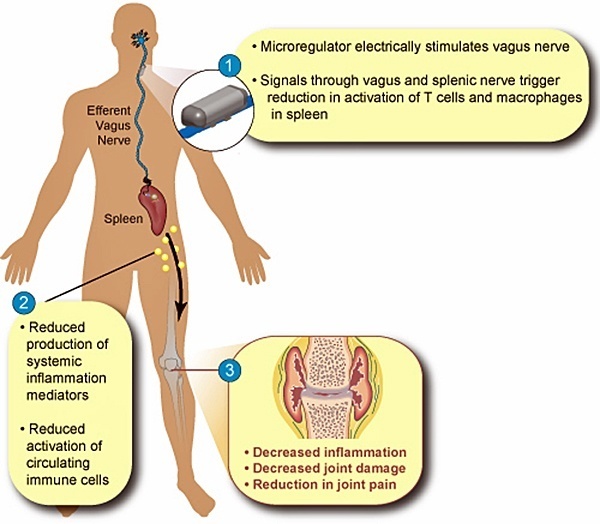
SetPoint Medical, headquartered in Valencia, California, is developing neuromodulation therapies for patients with inflammatory autoimmune diseases, such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD), psoriasis, diabetes, heart disease, and multiple sclerosis. SetPoint’s proprietary neuromodulation platform consists of an implantable “microregulator”, wireless charger and iPad prescription pad application.
SetPoint announced today that it has secured $27 million in financing from its current investors along with new investors Covidien Ventures, Action Potential Venture Capital Limited, the new GlaxoSmithKline (GSK) strategic venture capital fund for bioelectronic medicines and technologies, and Boston Scientific. The proceeds of the financing will be used to expand ongoing clinical development of the SetPoint bioelectronics therapy approach in rheumatoid arthritis and Crohn’s disease and advance development of the SetPoint proprietary neuromodulation platform.


 Start-up company
Start-up company  Boston Scientific announced that it has launched a clinical trial to determine whether occipital nerve stimulation (ONS) using the Precision™ System can safely and effectively treat chronic migraine when used in conjunction with anti-migraine medications. According to the press release:
Boston Scientific announced that it has launched a clinical trial to determine whether occipital nerve stimulation (ONS) using the Precision™ System can safely and effectively treat chronic migraine when used in conjunction with anti-migraine medications. According to the press release: Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently
Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently 
 NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.
NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.



![logo_mainstay1[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/logo_mainstay11.jpg)
![SynchroMed-II[1]](https://www.implantable-device.com/wp-content/uploads/2013/06/SynchroMed-II1-300x272.jpg)