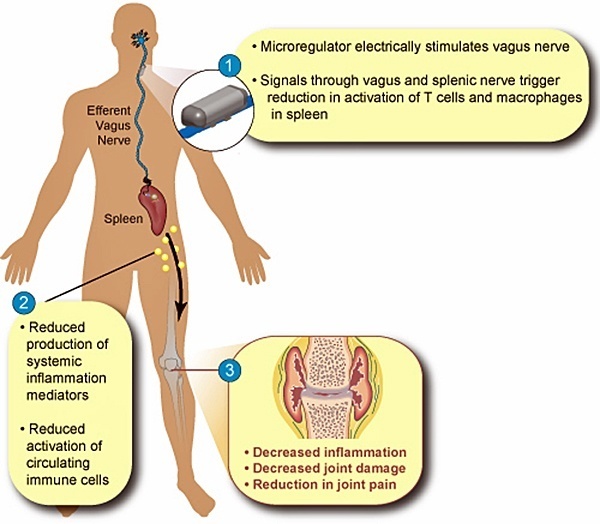
SetPoint Medical, headquartered in Valencia, California, is developing neuromodulation therapies for patients with inflammatory autoimmune diseases, such as rheumatoid arthritis (RA), inflammatory bowel disease (IBD), psoriasis, diabetes, heart disease, and multiple sclerosis. SetPoint’s proprietary neuromodulation platform consists of an implantable “microregulator”, wireless charger and iPad prescription pad application.

 Toronto-based
Toronto-based 
 St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.
St. Jude Medical announced it has received European CE Mark approval of its Eon™ family of neurostimulators for treating patients with intractable chronic migraine.

 Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.
Johns Hopkins’ Sridevi V. Sarma, an assistant professor of biomedical engineering, has devised new seizure detection software that, in early testing, significantly cuts the number of unneeded brain-stimulation therapy that an epilepsy patient would receive.


