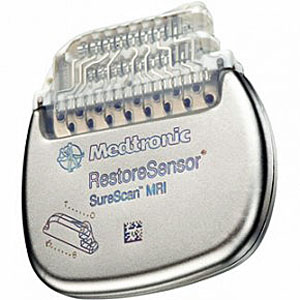
 Following Medtronic’s announcement in February of this year that it had introduced in Europe the first and only implantable neurostimulation systems indicated for use in the treatment of chronic back and/or leg pain that are designed for full-body Magnetic Resonance Imaging (MRI) scans under specific conditions, the Company announced today that FDA has approved the system for use in the US. Medtronic SureScan neurostimulation systems include enhancements to existing devices as well as specially designed leads to reduce or eliminate the hazards produced by the MRI environment. The devices also include a proprietary SureScan programming feature, which sets the device into an appropriate mode for the MRI environment.
Following Medtronic’s announcement in February of this year that it had introduced in Europe the first and only implantable neurostimulation systems indicated for use in the treatment of chronic back and/or leg pain that are designed for full-body Magnetic Resonance Imaging (MRI) scans under specific conditions, the Company announced today that FDA has approved the system for use in the US. Medtronic SureScan neurostimulation systems include enhancements to existing devices as well as specially designed leads to reduce or eliminate the hazards produced by the MRI environment. The devices also include a proprietary SureScan programming feature, which sets the device into an appropriate mode for the MRI environment.
The press release states:
“With the first U.S. implants of its new RestoreSensor® SureScan® MRI neurostimulation systems, Medtronic, Inc. (NYSE: MDT) is introducing the first and only implantable neurostimulation (also known as spinal cord stimulation, or SCS) systems for use in the treatment of chronic, intractable back and/or limb pain that are approved by the U.S. Food and Drug Administration (FDA) for conditionally safe* full-body Magnetic Resonance Imaging (MRI) under specific conditions.

 Start-up company
Start-up company 

 Boston Scientific announced that it has launched a clinical trial to determine whether occipital nerve stimulation (ONS) using the Precision™ System can safely and effectively treat chronic migraine when used in conjunction with anti-migraine medications. According to the press release:
Boston Scientific announced that it has launched a clinical trial to determine whether occipital nerve stimulation (ONS) using the Precision™ System can safely and effectively treat chronic migraine when used in conjunction with anti-migraine medications. According to the press release:
 Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently
Greatbatch Medical, which moved its headquarters to the Dallas, TX area last year, announced that it has set a target of at least 5%/yr organic growth. To accomplish this growth, the Company recently  On June 5, 2013 Greatbatch, Inc. announced that it would combine Greatbatch Medical and Electrochem Solutions – which have been operating independent operations and sales & marketing groups – into singular sales & marketing and operations groups serving the entire Greatbatch organization. According to the
On June 5, 2013 Greatbatch, Inc. announced that it would combine Greatbatch Medical and Electrochem Solutions – which have been operating independent operations and sales & marketing groups – into singular sales & marketing and operations groups serving the entire Greatbatch organization. According to the 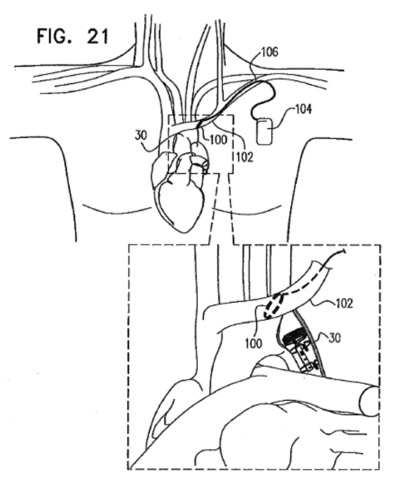
 NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.
NeuroTronik, a recent spin-off from Synecor, a Chapel Hill business accelerator, announced that it has raised $13.1M for the development of a neuromodulation system intended for the treatment of acute heart failure syndrome.

