
My new book is out and available through Amazon!
High-Power Near-Infrared Light Treatments for Depression, Dementia, and Other Brain Disorders
Light of specific wavelengths and intensities has been shown to repair and protect neurons from damage, opening the possibility of using near-infrared light as a non-invasive treatment of various brain and psychiatric disorders.
This book serves as a practical overview of the scientific fundamentals, technical implementation, and therapeutic applications of transcranial photobiomodulation:
- Part I provides an accessible explanation of why the irradiation must be done in the near-infrared region of the light spectrum. It presents evidence-based background on the parameters of light important to photobiomodulation, and describes the effects of near-infrared light on cells and tissues.
- Part II discusses the types of multi-Watt light sources required to non-invasively deliver therapeutic doses of infrared light to the brain. Importantly, this part describes in detail various High-Power Near-Infrared Transcranial Therapy (HIPNITT) medical devices that have been used in clinical trials.
- Part III presents the potential and challenges of using near-infrared light in the treatment of various brain disorders such as depression, dementia, traumatic brain injury, and Parkinson’s disease.
This book uses straightforward language and offers practical guidance to help readers quickly develop an understanding of the practical aspects of HIPNITT implementation and its therapeutic applications.











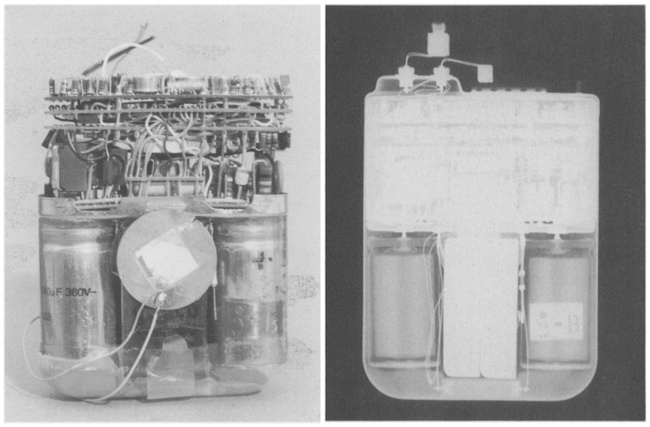

 Unlike Cyberonics’ VNS IPGs, the RNS® neurostimulator is designed to detect abnormal electrical activity in the brain and respond by delivering electrical stimulation to normalize brain activity before the patient experiences seizure symptoms. The neurostimulator is implanted in the cranium and connected to one or two leads that are implanted near the patient’s seizure focus.
Unlike Cyberonics’ VNS IPGs, the RNS® neurostimulator is designed to detect abnormal electrical activity in the brain and respond by delivering electrical stimulation to normalize brain activity before the patient experiences seizure symptoms. The neurostimulator is implanted in the cranium and connected to one or two leads that are implanted near the patient’s seizure focus.