
From today’s press release: “Improved performance in the company’s Cardiac Rhythm Management and Interventional Cardiology businesses, with CRM achieving growth of 1 percent on a constant currency basis.” Sales increased from $462M in Q3 2012 to $464M in Q3 2013, which represents a 1% change in constant-currency basis.


 Neuros Medical received an Investigational Device Exemption to conduct a pivotal clinical trial to evaluate the Altius™ System High Frequency Nerve Block technology for the management of intractable limb pain of amputees. The prospective, randomized, controlled pivotal clinical trial will consist of 130 patients at 15 institutions in the U.S. to evaluate the safety and efficacy of Neuros Medical’s Altius System.
Neuros Medical received an Investigational Device Exemption to conduct a pivotal clinical trial to evaluate the Altius™ System High Frequency Nerve Block technology for the management of intractable limb pain of amputees. The prospective, randomized, controlled pivotal clinical trial will consist of 130 patients at 15 institutions in the U.S. to evaluate the safety and efficacy of Neuros Medical’s Altius System.



 Net product sales increased 12.4% to $67.4 million in the first fiscal quarter ended July 26 for Cyberonics, Inc. of Houston, TX. Including license revenue, sales were up 14.2% overall, with Europe in particular contributing a strong performance. Diluted earnings per share were adjusted by $0.17 cents due to a litigation settlement.
Net product sales increased 12.4% to $67.4 million in the first fiscal quarter ended July 26 for Cyberonics, Inc. of Houston, TX. Including license revenue, sales were up 14.2% overall, with Europe in particular contributing a strong performance. Diluted earnings per share were adjusted by $0.17 cents due to a litigation settlement.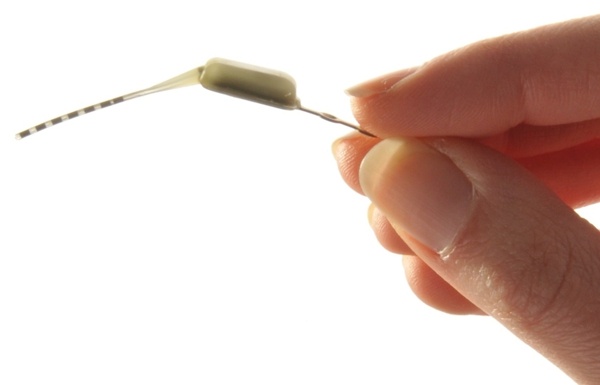
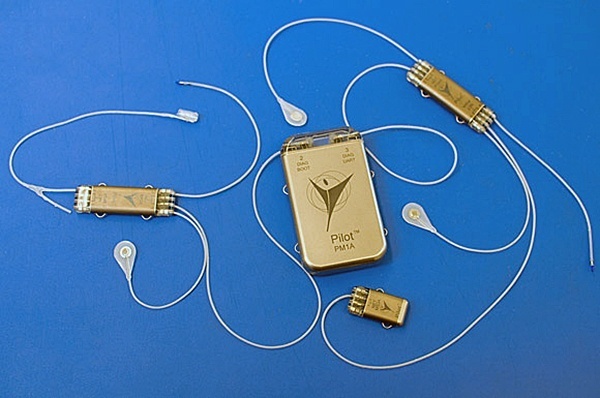
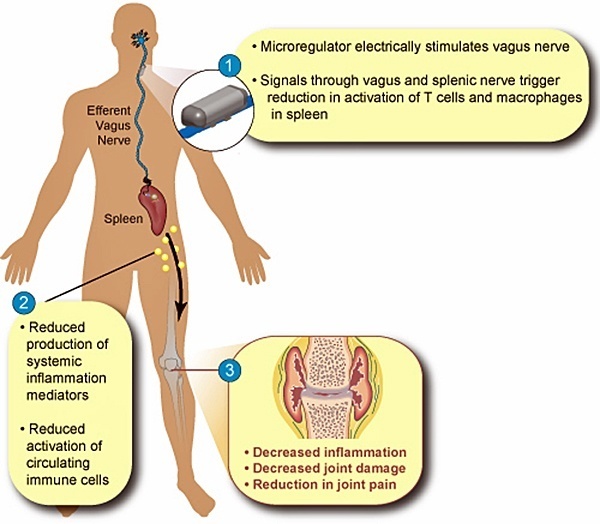

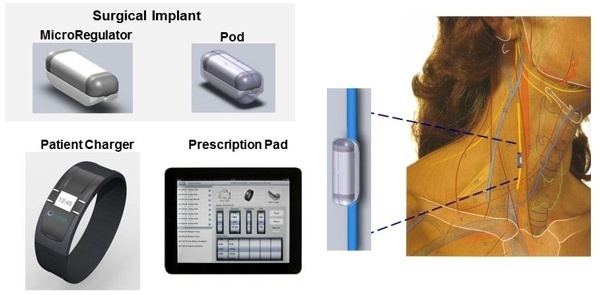
 Start-up company
Start-up company