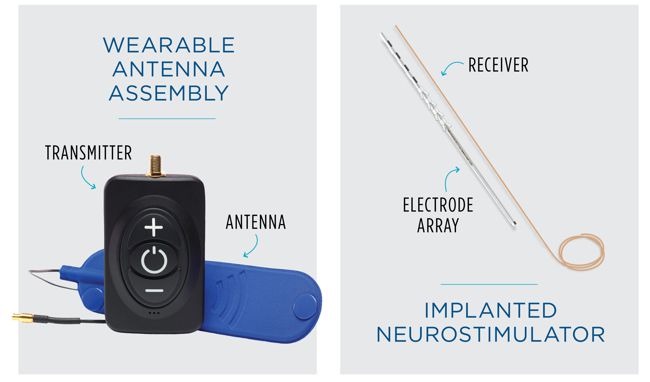
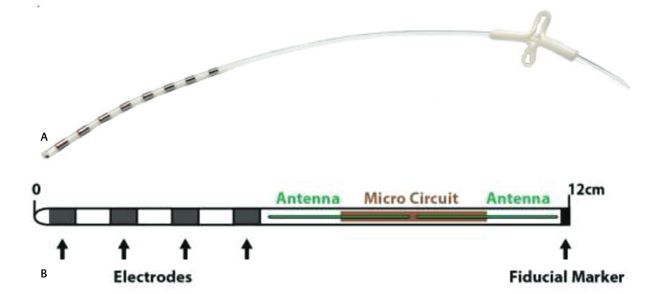
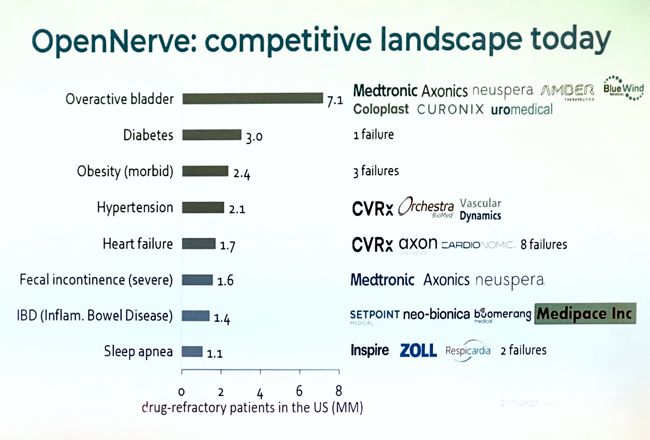
Image Credit: Aria CV
Aria CV, based in St. Paul, Minnesota, was founded in 2010 to develop an implantable device intended to treat pulmonary arterial hypertension.
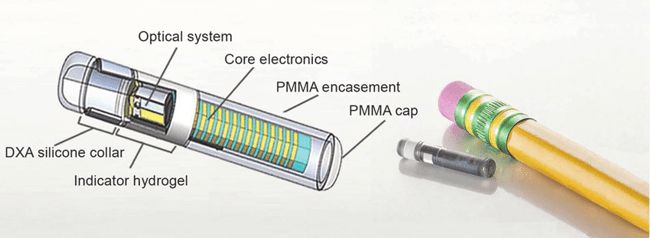
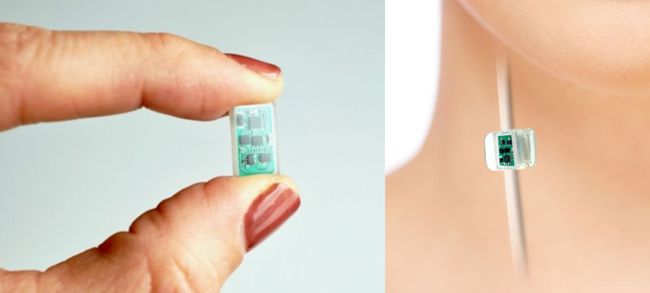
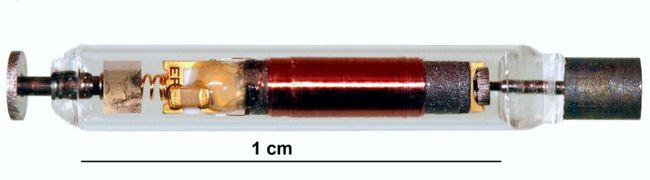
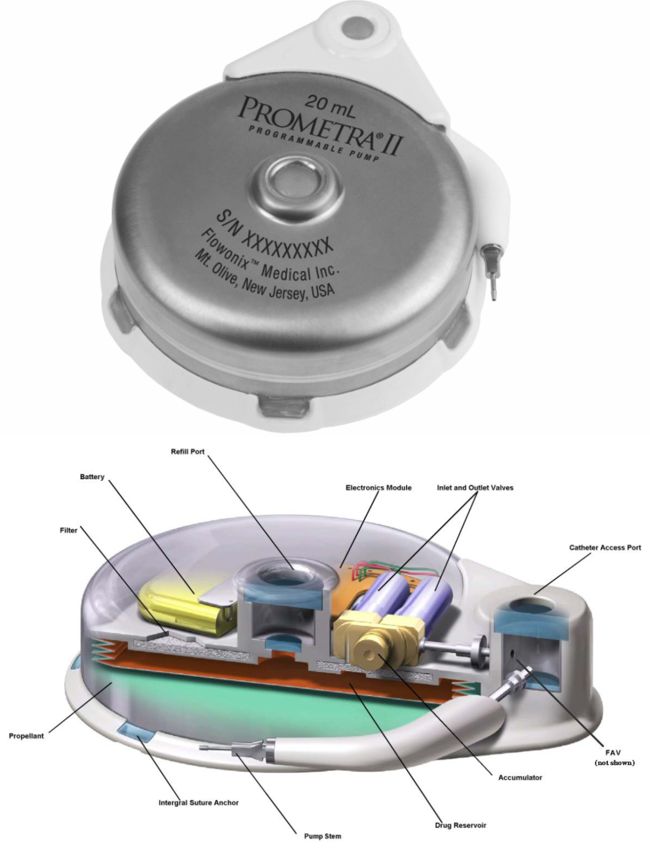
The Aria CV device is an implantable gas-filled balloon that is introduced percutaneously and functions in the main pulmonary artery to alleviate the excessive workload on the right heart that results in right heart failure. The device is designed to restore pulmonary artery health and elasticity, reducing the workload on heart muscles and increasing blood flow by mimicking the function of healthy vessels, which helps the heart pump at a more normal rate and improves blood flow to the lungs. Continue reading