Image Credit: BrainsGate
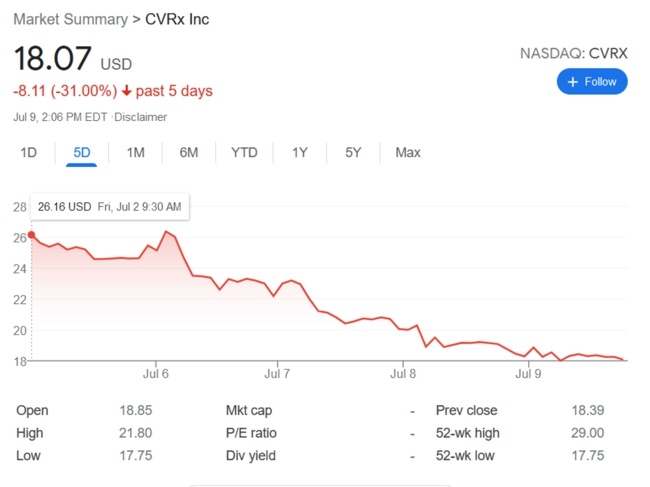
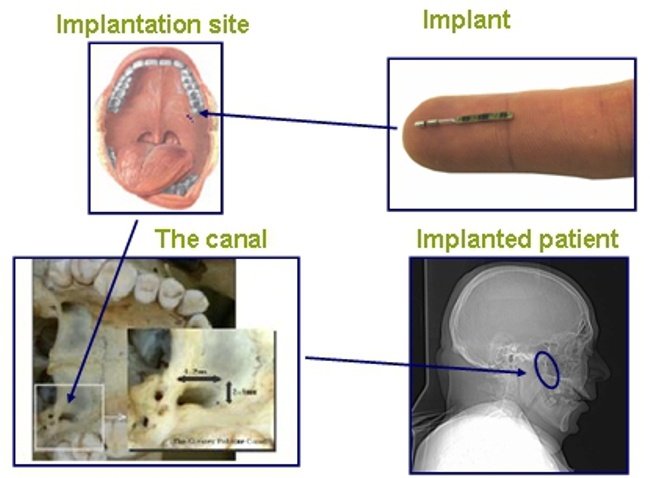
BrainsGate is an Israeli company that was established in 2000. It developed an implantable device for the stimulation of the Spheno-Palatine Ganglion (SPG) to modulate the brain-blood barrier and increase cerebral blood flow.
On December 10, 2021, BrainsGate’s clinical study was discussed by an FDA panel, which although agreed on the safety of the system, did not find the device sufficiently effective as a treatment for stroke, whereby its benefits would outweigh the risks.
The voting was:
- Safety: 13 votes in favor
- Effectiveness: 3 votes in favor, 7 against, 3 abstain
- Benefits outweigh risk: 3 votes in favor, 7 against, 3 abstain
FDA does not have to follow the recommendations of its panels, but it mostly does (approximately 80% of the time), and such low vote of confidence does not bode well for BrainsGate’s SPG stimulation as a treatment for ischemic stroke.
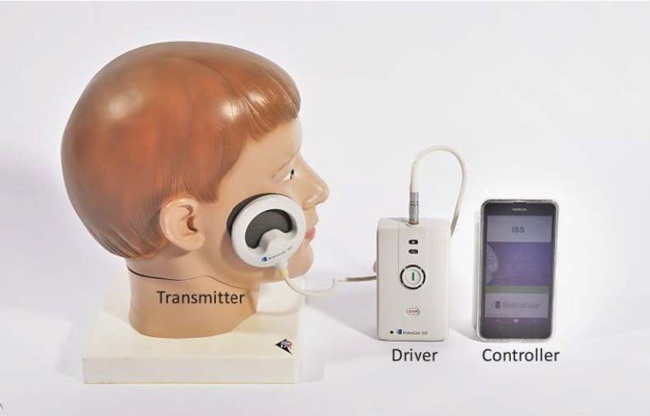
Image Credit: BrainsGate